Table of Contents
TL;DR
- Mechanistic evidence (2025): A Journal of Neuroinflammation study shows S1 (spike S1) enters astrocyte endolysosomes and triggers endolysosomal dysfunction and a senescence-like phenotype via TLR7 → p38 MAPK signalling. This demonstrates that persistent spike protein — even without viral RNA — can provoke a senescence-associated secretory phenotype (SASP) capable of sustaining neuroinflammation. J. Neuroinflammation 2025
- Cellular survival mechanisms: Spike protein may activate mTOR while inhibiting p53, enabling infected or transfected cells to survive and continue spike production instead of undergoing normal apoptosis.
- Lingering antigen: SARS-CoV-2 RNA can persist in multiple tissues months after mild disease; antigen detection associates with long-term symptoms in cohort data. (Chertow 2022; Zuo 2024, Lancet Infect Dis)
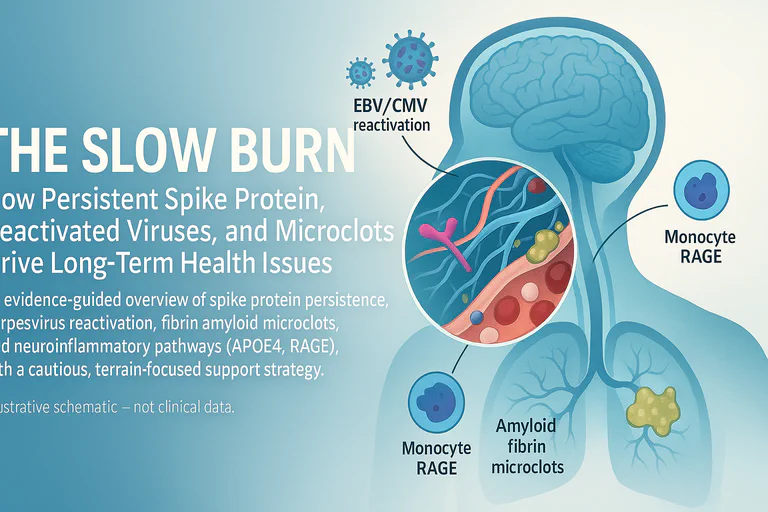
- Brain-borders persistence: Spike protein has been observed at the skull–meninges–brain axis in human autopsy tissues, with reports documenting presence up to 17 months post-vaccination. (Rong et al., 2024; Cell Host & Microbe; PMID 39615487; Ota et al., 2025; PMID 40184822)
- Post-vaccination syndrome (PVS): A 2025 preprint from the Yale LISTEN group reported elevated circulating spike protein in a subset of PVS participants compared to controls; one case noted up to 709 days post-vaccination; interpretation awaits peer review. (Bhattacharjee 2025, medRxiv)
- Immune tolerance mechanisms: RAGE/HMGB1 activation drives IL-10 production, creating a regulatory environment that blocks viral clearance—paralleling HIV tolerance paradigms.
- Viral reactivation: EBV/HSV/CMV reactivation is documented in COVID-19 (and occasionally after vaccination in case series/meta-analyses), with potential symptom impact. (Shafiee 2023; Boers 2024)
- Microclots & prion-like properties: Spike S1 can induce fibrinolysis-resistant amyloid(oid) fibrin in vitro and forms beta-sheet–rich fibrils that can accelerate aggregation of brain proteins (PrP, Aβ42). (Grobbelaar 2021; Pretorius 2021/2022; Svensson et al., 2025)
- Brain risk nodes: S1 can engage RAGE on monocytes; APOE4 genotype is linked to BBB leakiness and vulnerability to peripheral inflammation. (Angioni 2023; Montagne 2020; Marottoli 2017)
- Cumulative burden: Repeated exposures (infections or vaccinations) create additive damage; hazard ratio of 2.35 for ≥3 infections versus none, regardless of vaccination status. (Bowe et al., 2022)
- Holistic support: Nutrient-dense diets, oral-health focus, and Nrf2-activating foods (e.g., broccoli sprouts) are reasonable adjuncts—evidence for outcomes remains preliminary.
If we treated COVID-19 like a two-week respiratory blip, the research now paints a more chronic, multi-system picture. Think "slow burn": antigen persistence, immune perturbations, microvascular changes, and neurovascular stress—especially in vulnerable genotypes. Recent discussions, including endorsements from experts like Jessica Rose, highlight emerging evidence on brain-specific persistence. This article draws heavily from the foundational insights of Dr. Annelise Bocquet, whose detailed analyses on viral persistence and reactivation have been instrumental.
Part 1: Persistence isn't rare
Autopsy mapping detected viral RNA widely up to ~230 days post-infection. (Chertow et al., Nature 2022)
In mild cases, a single-centre cohort found tissue RNA/subgenomic RNA weeks–months after infection, and antigen detection associated with long-COVID symptoms. (Zuo et al., Lancet Infect Dis 2024)
Spike in cells/serum: S1 in non-classical monocytes up to ~15 months after infection (case-control). (Patterson 2022)
Brain-specific persistence: Human autopsy work reported S1 at the skull–meninges–brain interface. (Rong et al., Cell Host & Microbe 2024; PMID 39615487)
Brain endothelial cells: Spike protein detected in cerebrovascular endothelial cells, confirming blood-brain barrier penetration and cellular uptake. (Scholkmann & May 2023)
Cerebrospinal fluid detection: Spike S1 protein identified in CSF of post-vaccine individuals, demonstrating central nervous system penetration. (Yonker et al. 2023)
Mechanistic link (2025): A Journal of Neuroinflammation study shows S1 can be taken up into astrocyte endolysosomes and trigger endolysosomal dysfunction and a senescence-like secretory phenotype via TLR7 → p38 MAPK signalling — demonstrating that persistent spike protein alone (even without viral RNA) can provoke ongoing pro-inflammatory signalling at brain border tissues J. Neuroinflammation 2025
PVS preprint: LISTEN reported elevated circulating spike protein in a subset up to 709 days post-vaccination; causality/mechanism unresolved; peer review pending. (medRxiv 2025)
Takeaway: persistence signals exist, but prevalence and clinical meaning differ by cohort and method, and in the context of vaccination, are still being defined in humans. Avoid absolutist language until multi-centre replication matures.
The Cellular Survival Paradox: Why Spike-Producing Cells Don't Die
One of the most critical questions in spike persistence is: Why do cells continue producing spike protein for months when they should have been cleared?
Recent mechanistic insights reveal a troubling answer: spike protein may hijack fundamental cell survival pathways.
The mTOR/p53 Survival Mechanism
Analysis by researchers examining persistent spike production proposes that SARS-CoV-2 spike protein activates mTOR (mechanistic target of rapamycin) while simultaneously inhibiting p53, the cell's "guardian of the genome":
- mTOR activation: Promotes cell growth, protein synthesis, and metabolic reprogramming — keeping the cell "alive and productive"
- p53 inhibition: Blocks apoptosis (programmed cell death) and DNA damage responses — preventing the cell from self-destructing
- Net result: Cells that should die continue to survive and produce spike protein
Clinical relevance: This mechanism could explain both viral reservoirs after infection AND prolonged spike production after vaccination, as transfected cells that would normally undergo apoptosis after fulfilling their immunogenic role instead persist.
Supporting evidence:
- Ota et al. (2025) documented spike protein in cerebral arterial walls 17 months post-vaccination in hemorrhagic stroke cases. (PMID: 40184822)
- Patterson et al. (2024) found S1 in monocytes up to 245 days post-vaccination. (medRxiv)
- Video analysis: WeirdSauce interview on mTOR/p53 mechanisms
Part 2: Immune drift & latent virus wake-ups
- Regulatory/tolerogenic shifts are described in chronic viral states (e.g., HIV Treg/IDO paradigms) and are hypothesized in post-acute COVID contexts. (Jenabian 2014)
- Senescence & SASP as an immune driver: Persistent antigen and chronic innate sensing (now directly shown for S1 in astrocytes via TLR7→p38 MAPK) can induce a senescence-associated secretory phenotype (SASP). SASP factors (IL-6, IL-1β, chemokines) shift local immunity toward chronic innate activation and regulatory feedback loops that can blunt effective adaptive clearance and promote immune drift.
The Immune Tolerance Trap: RAGE, HMGB1, and IL-10
Beyond the TLR7→p38 MAPK pathway, spike protein triggers a parallel tolerance mechanism that may explain why some individuals cannot clear the virus or antigen:
The RAGE/HMGB1/IL-10 Cascade
- RAGE activation: Spike S1 engages RAGE (Receptor for Advanced Glycation End-products) on monocytes and endothelial cells
- HMGB1 release: RAGE activation triggers High Mobility Group Box 1 protein release — a damage-associated molecular pattern (DAMP)
- IL-10 production: The RAGE/HMGB1 axis drives regulatory cytokine IL-10, creating an anti-inflammatory, tolerogenic environment
- ACE2 overexpression: IL-10 paradoxically upregulates ACE2 (the viral receptor), facilitating viral expansion
- Blocked clearance: Regulatory T-cell expansion and IL-10 dominance prevent effective viral/antigen elimination
Parallels to HIV tolerance: This mechanism mirrors HIV-associated immune tolerance, where persistent antigen drives IL-10/Treg dominance and progressive immune exhaustion. (PMID: 39615487)
Why asymptomatic COVID matters: Some individuals with high IL-10/regulatory responses may remain asymptomatic carriers with ongoing viral replication — a state of "immune ignorance" rather than clearance.
Vaccination-Induced Immune Drift
mRNA vaccination induces similar cytokine profile shifts that parallel early stages of the immune tolerance trap:
- IL-10 elevation alongside IL-6, IL-6R, TNF-α, and IL-1β changes post-vaccination
- Dose-dependent effect: Cytokine alterations markedly higher after 2-3 doses versus single dose
- microRNA dysregulation: miR-21-5p↑ (TLR/NF-κB regulator), miR-23a-3p↓ (NF-κB suppressor), miR-451a↑ (erythroid stress marker)
- Peak timing: 5-6 weeks post-vaccination — overlapping with window for post-vaccine syndrome onset
Critically, these molecular changes occurred independently of antibody levels, suggesting vaccination itself can initiate immune drift patterns that mirror early tolerance trap mechanisms (PMC11209245).
Clinical relevance: Repeated vaccination may compound pre-existing infection-induced immune dysregulation, potentially accelerating progression toward the tolerance trap state in susceptible individuals.
- Herpesviruses: Meta-analyses/case series document EBV/HSV/CMV reactivation with COVID-19; ICU HSV reactivation has been linked to worse outcomes. (Shafiee 2023; Boers 2024)
- Mechanistic interplay: SASP cytokines, impaired antigen presentation (MHC-I dysregulation reported for ORF8/ORF7a in vitro), and chronic monocyte/endothelial activation create a permissive environment for latent herpesviruses to reactivate — which in turn fuels more inflammation in a feed-forward cycle.
- Transplant/relapse cases underscore risks under profound immunosuppression and show how reactivation can be clinically consequential. (Cureus 2025; PMID 40747163; PMCID PMC12311555)
Takeaway: Immune drift in post-acute states likely reflects interacting processes — persistent antigen, senescent cellular secretomes, dysregulated innate sensing and occasional viral reactivation — rather than a single linear cause. This complexity explains heterogeneity in patient presentations and responses to interventions.
Part 3: Microclots & oxygen delivery
- In vitro S1 drives amyloid-like fibrin(ogen) resistant to fibrinolysis. (Grobbelaar 2021)
- Long-COVID studies report fibrin amyloid microclots and platelet hyperactivation, with proteomic cargo that could sustain inflammation and hinder microcirculation. (Pretorius/Kruger 2021–2022, open-access review)
- Mechanistic bridge (persistence → microclots): Senescent glia and persistently activated monocytes release SASP cytokines and pro-thrombotic signals (IL-6, tissue factor pathway activators, complement fragments) that promote endothelial activation and platelet hyperreactivity. Endothelial dysfunction plus a fibrin(ogen) structural shift toward amyloid(oid) assemblies creates a milieu where microclots form and persist, reducing effective microvascular oxygen delivery.
Prion-Like Properties: When Spike Becomes a Seeding Template
Beyond its direct effects on fibrin, the S1 subunit itself exhibits troubling prion-like characteristics:
S1 as an Atypical Non-Conventional Prion (ATNC)
Biochemical evidence suggests S1 behaves like a prion — a misfolded protein that templates further misfolding:
- Amyloid fibril formation: S1 forms beta-sheet–rich, fibrillar structures resistant to degradation. (Svensson et al., 2025, ACS Biochem; DOI: 10.1021/acs.biochem.5c00550)
- Accelerated neurodegeneration: Spike S1 accelerates aggregation of human prion protein (HuPrP) and amyloid-beta 42 (Aβ42) in vitro. (Idrees & Sohail, 2023 bioRxiv preprint)
- HIF-1α/p53 synergy: Hypoxia-inducible factor 1-alpha (HIF-1α) interacts with prion protein pathways, potentially linking tissue hypoxia (from microclots) with accelerated protein misfolding. (Jeong et al., 2011; PMID: 22036844)
- ORF6 contribution: SARS-CoV-2 ORF6 protein contains amyloidogenic sequences that may contribute to aggregate formation. (Sprunger & Jackrel, 2023)
Fibrin–Spike Synergy: The "Calamari Clots"
The interaction between spike and fibrinogen creates hybrid amyloid structures:
- Spike binds fibrinogen directly, inducing conformational changes
- Resulting fibrils display both spike epitopes AND amyloid characteristics
- These aggregates mimic prion "plaques" — resistant to normal proteolysis
- Pretorius et al. termed these "calamari clots" for their unusual rubbery, fibrous morphology
Unified framework: The tolerogenic (IL-10), allergenic (RAGE/mast cell activation), and potentially carcinogenic (HIF-1α/p53 dysregulation) aspects of spike persistence may converge via prion-like mechanisms — creating a framework for understanding multi-system, progressive Long COVID symptoms.
- Clinical significance: The presence of microclots correlates with symptoms in several cohorts, but standardisation of assays, causal proof, and interventional data are still required. Small open studies suggest benefit from antiplatelet/antithrombotic strategies in select patients, but robust RCTs are missing.
Takeaway: Persistent antigen → chronic innate signalling → endothelial & platelet activation → prion-like aggregation is a plausible path to microvascular impairment and neurodegeneration; proving causality and safe, effective treatments remains an active research priority.
Part 4: Neurovascular nodes — APOE4, RAGE, BBB
The breakdown of barrier integrity—whether in the gut, oral cavity, or blood-brain barrier—is a recurring theme in chronic inflammatory conditions. Research into endotoxemia and systemic inflammation, explored in contexts ranging from sepsis to complex neuroimmune conditions, provides a valuable framework for understanding the multi-system symptoms seen in Long COVID. In this model, a persistent trigger (like spike protein) initiates a cascade of barrier breaches, allowing microbial components (like LPS from the gut or oral cavity) to fuel a chronic, systemic inflammatory state that ultimately converges on the brain's vascular defenses.
- RAGE axis: Multi-omics and cellular work indicate S1–RAGE engagement on monocytes and endothelial cells; the nucleocapsid protein may also interact with RAGE pathways. (Angioni 2023)
- Astrocyte mechanism (2025): A Journal of Neuroinflammation study shows S1 is taken into astrocyte endolysosomes and causes endolysosomal dysfunction that triggers a TLR7 → p38 MAPK cascade, producing a senescence-like phenotype and SASP (IL-6, etc.). This provides a direct cellular route from persistent spike to chronic neuroinflammatory signalling at brain border tissues. (J. Neuroinflammation 2025)
- APOE4 & BBB: APOE4 carriers show hippocampal BBB breakdown independent of Aβ/tau, predicting cognitive decline and vulnerability to peripheral inflammation. (Montagne 2020)
- Peripheral inflammation × APOE4: Systemic LPS or chronic peripheral inflammation exacerbates BBB leak and cognitive deficits in E4/Aβ-prone models — a conceptual parallel to how chronic SASP/monocyte activation could amplify neurovascular injury. (Marottoli 2017)
- Oral–immune link & Metabolic Endotoxemia: This oral–immune link creates metabolic endotoxemia. Oral pathogens like Prevotella intermedia release proteases that degrade CD14 and LBP, critical regulators of endotoxin (LPS). This impairment allows LPS to enter systemic circulation, where it drives chronic inflammation, insulin resistance, and endothelial dysfunction—a pathological triad that mirrors key features of Long COVID. (Deschner 2003; Andrukhov 2016)
Synthesis: The mechanistic chain to consider is a multi-barrier cascade:
persistent S1 → TLR7 sensing (astrocytes/monocytes) → p38 MAPK activation → endolysosomal dysfunction → senescence/SASP → endothelial & immune activation → BBB stress / microvascular injury. This central nervous system impact is dramatically amplified by concurrent gut/oral barrier dysfunction, which leads to metabolic endotoxemia, creating a state of chronic peripheral inflammation that relentlessly attacks the blood-brain barrier. In APOE4 carriers or people with metabolic dysfunction, this feed-forward loop plausibly accelerates symptomatic decline.
Takeaway: Brain-border persistence plus genetic vulnerability and systemic inflammation create intersecting risk nodes for chronic neurovascular and cognitive dysfunction.
A Note on Scientific Context: The investigation into immune and barrier dysfunction in chronic conditions has a complex history. In other fields, hypotheses linking endotoxemia and barrier integrity to chronic symptoms have faced significant controversy and required decades of rigorous research to reach consensus. This historical context underscores the need for cautious interpretation of preliminary findings in Long COVID and the critical importance of well-designed clinical trials to avoid premature conclusions.
Practical biomarkers to track BBB integrity & neuroinflammation
Tracking these biomarkers over time provides a quantifiable window into blood–brain barrier integrity, neuroinflammatory tone, and systemic-to-central crosstalk.
How to use: repeat every 8–12 weeks while symptoms evolve; look for directional trends, not single "perfect" numbers.
| Panel | Biomarker | Why it helps (one-liner) |
|---|---|---|
| Core | CRP (hsCRP), fibrinogen | Systemic inflammation & clotting tendency |
| D-dimer | Ongoing fibrin turnover / microthrombi signal | |
| Ferritin, CBC (platelets) | Inflammatory load; platelet activation context | |
| ALT/AST, GGT | Metabolic/ROS stress background | |
| Neuro/BBB | GFAP, sNfL | Astrocytic & axonal injury load over time |
| S100B (if available) | BBB leak proxy in some contexts | |
| Endothelium/Coag | VWF:Ag, ADAMTS13 ratio | Endothelial stress / microangiopathy signal |
| Fibrin-monomer / thrombin time (lab-dependent) | Pro-coagulant milieu | |
| Barrier/Gut–Oral | LPS-binding protein (LBP), sCD14 | Endotoxin exposure/handling |
| Zonulin (caveats) | GI barrier modulation (assay variability) | |
| Metabolic add-ons | fasting insulin, TG/HDL, HbA1c | Insulin resistance & vascular risk context |
| Antigen persistence | Anti-S/Anti-N antibody ratios | Differentiates infection vs vaccination; tracks antigen exposure timeline |
| Spike protein mass spectrometry | Direct detection of circulating spike fragments (research-grade) | |
| Immune tolerance | IL-10 levels | Marks regulatory/tolerogenic shift |
| Treg frequency (flow cytometry) | Quantifies regulatory T-cell expansion | |
| Prion-like pathology | Thioflavin T fluorescence (blood smear) | Detects amyloid fibrils in circulation (research protocol) |
| Nailfold capillaroscopy | Visualizes fibrinoid deposits in microvasculature |
Clinical decisions belong with your clinician; this is informational context only.
Note on availability: Some advanced markers (spike mass spec, Thioflavin T staining, nailfold capillaroscopy) are research-grade or available only through specialized labs (e.g., Synaptek Labs); they are not standard clinical practice yet. A comprehensive diagnostic framework for spike persistence has been proposed. (Halma & Varon, 2025, Frontiers Med)
Microbiome / Biofilms"] -->|LPS / PGN| LBP["LBP → sCD14
Biomarkers: LBP, sCD14, Zonulin"] LBP --> MONO["Monocytes / Endothelium"] MONO -->|RAGE / TLRs| NFkB["NF-κB / Cytokines
Biomarkers: CRP, Ferritin, CBC"] NFkB --> COAG["Coagulation Shift
Fibrinogen ↑ • D-dimer ↑
Biomarkers: Fibrinogen, D-dimer, VWF/ADAMTS13, Fibrin-monomer"] COAG --> MICRO["Microclots / ↓ O₂ Delivery
Biomarkers: D-dimer"] NFkB --> BBB["BBB Stress
GFAP ↑ • S100B ↑
Biomarkers: GFAP, S100B"] MICRO --> CNS["Neuro Symptoms / Fatigue / Cog
Biomarkers: sNfL"] BBB --> CNS subgraph Tracking Panel CRP["CRP (hsCRP)"] FIB["Fibrinogen"] DD["D-dimer"] GFAPn["GFAP"] SNFL["sNfL"] S100Bv["S100B"] VWFn["VWF:Ag / ADAMTS13"] LBPn["sCD14 / LBP / Zonulin"] Ferritin["Ferritin / CBC"] ALTAST["ALT/AST, GGT"] Metabolic["Fasting Insulin, TG/HDL, HbA1c"] end CRP -.-> NFkB Ferritin -.-> NFkB ALTAST -.-> NFkB FIB -.-> COAG DD -.-> MICRO GFAPn -.-> BBB S100Bv -.-> BBB SNFL -.-> CNS VWFn -.-> COAG LBPn -.-> LBP Metabolic -.-> COAG
Terrain-centric support (adjunctive, not curative) — expanded with mechanistic rationale
- Diet quality & polyphenols: Reasonable bet for inflammation/oxidative-stress modulation; mechanistically these foods can reduce NF-κB signalling and bolster antioxidant defences. Clinical outcome evidence in PASC/PVS is limited. (Review 2024)
- Oral health & biofilms: Treating periodontitis reduces systemic inflammatory burden in other contexts; given the oral→LBP/sCD14 axis, improving oral hygiene is low-risk and plausibly beneficial.
- Nrf2 activators: Sulforaphane-rich foods plausibly reduce oxidative stress and downstream endothelial activation — mechanistically attractive but unproven for PASC outcomes.
- Sleep, stress, graded activity: Reduce sympathetic drive, cortisol dysregulation and inflammation — low-risk bedrock measures.
- Mechanistically-informed (experimental) targets: The J. Neuroinflammation TLR7→p38→senescence result points to several hypothesis-generating interventions:
- p38 MAPK modulation: p38 inhibitors have been explored in inflammatory diseases; they are a mechanistic candidate but carry safety and off-target concerns.
- TLR7 pathway dampening: TLR7 antagonism could reduce persistent innate signalling but risks impairing anti-viral defence — clinical trial data are needed.
- Senolytics / senomorphic agents: Removing or modulating senescent cell burden (or reducing SASP) is theoretically attractive to break chronic inflammatory loops; human data in PASC are currently absent and safety must be evaluated.
- Anti-coagulant/antiplatelet strategies: Small, uncontrolled reports suggest symptom benefit in subsets with microclot signals; these approaches require careful clinical oversight and RCT validation.
- Practical medical advice: Any use of experimental or off-label pharmacologic agents must be undertaken by clinicians in the context of informed consent and, where possible, clinical trials.
Disclaimer: These mechanistic targets are hypothesis-generating and not clinical recommendations. Trial evidence is required before routine clinical use.
Therapeutic Implications: Targeting mTOR & Autophagy for Spike Clearance
Given the emerging evidence that spike protein hijacks mTOR signaling to promote cellular survival, researchers have begun exploring whether mTOR inhibitors and autophagy inducers could help clear persistent spike and restore normal cellular function.
The mTOR Connection: Why Targeting This Pathway Matters
The mechanistic target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates:
- Cell growth and proliferation
- Protein synthesis
- Metabolism
- Autophagy — the cell's recycling system
The problem: Spike protein appears to activate mTOR while inhibiting p53, creating a survival advantage for spike-producing cells that should normally undergo apoptosis.
The solution: By inhibiting mTOR, we may:
- Reactivate autophagy — clearing spike protein and damaged cellular components
- Induce apoptosis in persistently infected/transfected cells
- Reduce viral replication (mTOR is hijacked by many viruses)
- Modulate immune responses — reducing chronic inflammation while enhancing antiviral T-cells
mTOR Inhibitors: Research Status
Rapamycin (Sirolimus) and Analogs
What they do:
- Form complex with FKBP12 to inhibit mTORC1
- Promote autophagy by relieving ULK1 inhibition
- Suppress protein synthesis (hindering viral production)
- Enhance stem-like CD8+ T-cells and reduce exhaustion
Evidence in COVID-19 context:
- Preclinical studies: Rapamycin restricts SARS-CoV-2 replication in cell culture
- Kidney transplant patients: On rapamycin showed reduced severity of COVID-19 and lower incidence of pulmonary fibrosis
- Aging resilience: Low-dose rapamycin improves IFN-induced immunity in older adults
- Long COVID hypothesis: By inducing autophagy, could help clear persistent spike remnants

The mTOR pathway integrates signals from nutrients, growth factors, and energy status to regulate cell growth and autophagy. Spike protein hijacks this pathway for persistence.
Safety considerations:
- Can cause mouth sores, hyperglycemia, and immunosuppression
- Timing is crucial — early inhibition may aid viral clearance, but in immunocompromised patients may increase infection risk
- Not available without prescription; clinical trials are required before routine use
Next-Generation mTOR Inhibitors
Drugs like sapanisertib and vistusertib target the kinase domain directly and inhibit both mTORC1 and mTORC2, offering broader effects but with potentially increased side effects.

The PI3K-AKT-mTOR axis is hyperactive in many cancers and is hijacked by viruses. Targeting this pathway may have dual benefits in spike persistence and cancer prevention.
Autophagy: The Body's Cleanup System
What is autophagy? A fundamental cellular process where cells encapsulate damaged components or pathogens in double-membrane vesicles (autophagosomes) that fuse with lysosomes for degradation.
How it helps clear spike:
- Direct degradation (Xenophagy): Autophagy receptors recognize ubiquitinated spike protein and guide it into autophagosomes for breakdown
- Capsid disassembly: During viral entry, autophagy facilitates breakdown of viral proteins
- Immune modulation: Autophagy fine-tunes interferon production and presents viral antigens to T-cells
The double-edged sword: Some viruses (including SARS-CoV-2 via ORF3a) can block autophagosome-lysosome fusion, using autophagic structures for replication. This means restoring autophagic flux (not just inducing it) is critical.
Natural Compounds That Induce Autophagy
Several well-studied natural compounds activate autophagy through mTOR-independent pathways:
1. Spermidine
- Found in aged cheese, wheat germ, soy products, mushrooms
- Induces autophagy by inhibiting acetyltransferase EP300
- Shown to extend lifespan in animal models
- Human data: Associated with reduced cardiovascular mortality and cognitive decline
2. Resveratrol
- Activates sirtuin pathways (SIRT1) which promote mitochondrial biogenesis
- Induces autophagy via AMPK activation and mTOR inhibition
- Evidence: Improves endothelial function and reduces oxidative stress in clinical trials
3. Quercetin
- Polyphenol flavonoid found in onions, apples, berries
- Activates autophagy via TFEB (transcription factor EB) nuclear translocation
- COVID-specific data: Small RCTs suggest reduced inflammatory markers
4. Curcumin
- Induces autophagy through multiple pathways including mTOR inhibition
- Evidence: Anti-inflammatory effects documented, but bioavailability is poor
5. EGCG (Epigallocatechin Gallate)
- From green tea
- Activates autophagy via AMPK/mTOR axis
- COVID relevance: Shown to inhibit SARS-CoV-2 main protease in vitro
6. HIF-1α Stabilizers
- Natural compounds that stabilize hypoxia-inducible factor 1-alpha (HIF-1α)
- Have shown efficacy against viruses like Japanese encephalitis by boosting autophagic flux
- Potential application: May help clear spike while supporting cellular adaptation to hypoxia from microclots
Therapeutic Approaches Under Investigation
| Approach | Mechanism | Evidence Status | Notes |
|---|---|---|---|
| Rapamycin/Everolimus | mTORC1 inhibition → autophagy induction | Preclinical + transplant cohort data | Prescription-only; requires monitoring |
| Spermidine supplementation | EP300 inhibition → autophagy | Animal + observational human data | Generally recognized as safe (GRAS) |
| Nattokinase/Lumbrokinase | Fibrinolytic + may support clearance | In vitro + small pilot studies | May work synergistically with autophagy |
| Polyphenol combinations | Multi-pathway autophagy activation | Small RCTs for inflammation | Bioavailability challenges |
| Intermittent fasting | Natural autophagy induction | Strong animal data, human studies ongoing | Time-restricted eating shows promise |
| NAD+ precursors (NR/NMN) | SIRT activation → autophagy + mitochondrial health | Early human trials for aging | May support energy metabolism in chronic fatigue |
Key Open Questions
- Dosing and timing: What is the optimal window for mTOR inhibition after COVID/vaccination?
- Patient stratification: Who benefits most? (e.g., those with demonstrated spike persistence via LC-MS)
- Combination therapies: Could mTOR inhibitors + autophagy inducers + fibrinolytics work synergistically?
- Biomarker monitoring: How do we track autophagic flux in clinical practice? (LC3-II/I ratios, p62 degradation in research settings)
Clinical Trial Landscape (2025-2026)
As of 2025, several trials are exploring:
- Low-dose rapamycin for Long COVID
- Spermidine-rich diets for post-viral fatigue
- Combination approaches (autophagy inducers + antivirals)
Important caveat: While the mechanistic rationale is strong, clinical outcome data are still pending. Most interventions remain investigational for spike persistence syndromes.
Evidence roundup (curated)
Spike Persistence & Cellular Mechanisms
- Tissue persistence & long-COVID association: Zuo et al., 2024 (Lancet Infect Dis, PMID 38663423)
- Autopsy mapping to 230 days: Chertow et al., 2022 (Nature, PMID 36517603)
- S1 in monocytes up to 15 months: Patterson et al., 2022 (Front Immunol, PMID 35082777)
- S1 in monocytes up to 245 days post-vaccination: Patterson et al., 2024 (medRxiv)
- Skull–meninges–brain spike persistence: Rong et al., 2024 (Cell Host & Microbe, PMID 39615487)
- Spike in cerebral arteries 17 months post-vaccination: Ota et al., 2025 (PMID 40184822)
- Brain endothelial cell spike detection: Scholkmann & May 2023
- Cerebrospinal fluid spike protein: Yonker et al. 2023
- Lymph node persistence (60 days): Buergin et al. 2023
- Myocardial spike persistence (30 days): Bennett et al. 2024
- PVS spike up to 709 days (preprint): Bhattacharjee et al., 2025 (medRxiv)
- PACVS/PACS biomarker framework: Halma & Varon, 2025 (Frontiers Med)
Immune Tolerance & Viral Reactivation
- HIV-like tolerance mechanisms: Jenabian 2014 (PMC4125509)
- Herpesvirus reactivation (COVID & post-vax reports): Shafiee et al., 2023 (PMID 37559096); Boers et al., 2024 (PMID 39017695)
Microclots & Prion-Like Pathology
- S1-induced amyloid fibrin in vitro: Grobbelaar et al., 2021 (PMID 34328172)
- Long-COVID microclots & proteomics: Pretorius/Kruger 2021–2022 (PMID 34425843)
- Spike-induced amyloid fibrils: Svensson et al., 2025 (ACS Biochem)
- Spike accelerating prion/Aβ42 aggregation: Idrees & Sohail, 2023 (bioRxiv preprint)
- HIF-1α/PrP interactions: Jeong et al., 2011 (PMID 22036844)
- ORF6 amyloidogenic sequences: Sprunger & Jackrel, 2023 (ACS)
RAGE, BBB & Neurovascular Risk
- RAGE pathway & spike S1: Angioni et al., 2023 (Cell Rep Med, PMID 37944530)
- APOE4 → BBB leak & decline: Montagne et al., 2020 (Nature, PMID 32376954)
- LPS worsens E4/Aβ model cognition & BBB: Marottoli et al., 2017 (PMID 28707482)
Oral-Immune & Barrier Dysfunction
- P. intermedia proteases cleave CD14/LBP; sCD14 boosts LPS signaling: Deschner 2003 (PMID 12728301); Andrukhov 2016 (PMID 27135432)
Cumulative Damage
- Reinfection risks regardless of vaccination: Bowe et al., 2022 (Nature)
mTOR Inhibitors & Autophagy (NEW 2025-2026)
- mTOR pathway and spike persistence: Melo et al., 2025 (Viruses, PMID 40431629)
- Rapamycin restricts SARS-CoV-2 replication: Multiple studies 2022-2025
- mTOR inhibitors in kidney transplant COVID outcomes: Observational cohort data 2020-2024
- Autophagy in viral clearance: Review 2025
- Spermidine extends lifespan via autophagy: Eisenberg et al., Nature 2016
- Natural autophagy inducers (resveratrol, quercetin, EGCG): Multiple reviews 2020-2025
Acknowledgments: A Special Thanks to Open-Source Pioneers
In the spirit of collaborative science and open discourse, this article owes a profound debt to the tireless work of Dr. Annelise Bocquet and Dr. Jessica Rose. Both have generously shared their expertise through public platforms, demystifying complex topics like viral persistence, immune dysregulation, and post-vaccination effects for a global audience.
Dr. Bocquet, a Doctor in Health Biology and educator in hematology/immunology, has provided invaluable threads and analyses on X, challenging assumptions and fostering discussion with her mantra: "In science, we can affirm nothing, but we can discuss everything." Her insights into SARS-CoV-2 latency, spike persistence, and reactivation have been foundational to this piece.
Similarly, Dr. Jessica Rose, through her X posts and Substack "Unacceptable Jessica," has amplified critical data on vaccine safety, persistence, and long-term health impacts, often with a blend of rigor and accessibility that empowers readers to engage deeply.
By open-sourcing their hard work—via threads, articles, and public commentary—they've advanced public understanding and encouraged evidence-based dialogue. Thank you both for your courage, dedication, and commitment to transparency. Your contributions remind us that science thrives in the open.
Key Resources
- Nature - Tissue Persistence
- Frontiers - Monocyte Spike
- Lancet - Mild COVID Persistence
- Cell Host Microbe - Brain Spike Persistence
- PNAS Nexus - APOE4 & Prevotella (exploratory association)
- Pretorius Video - Microclots
- X - Nicholas Fabiano Thread on Brain Persistence
- X - Jessica Rose (JesslovesMJK) Endorsement
- Jessica Rose's Substack - Unacceptable Jessica
- X - Jessica Rose (@JesslovesMJK)
- X - Annelise Bocquet (@AnneliseBocquet)
- X - Annelise Bocquet on SARS-CoV-2 Latency/Reactivation
- X - Annelise Bocquet on Spike Persistence and Immunodeficiency
- X - Annelise Bocquet on Fatal SARS-CoV-2 Reactivation
- Thread Reader App - Annelise Bocquet's Threads
- X - Genome Defense
- WeirdSauce - mTOR/p53 in spike persistence
- Frontiers - PACVS/PACS Biomarker Framework
Media & Commentary (clearly non-primary)
These links are useful for context/discussion but are not primary evidence. Keep them separate from references.
- PNAS Nexus — APOE4 & Prevotella (exploratory association)
- Pretorius — Microclots (video overview)
- Thread Reader — Annelise Bocquet's Threads
- Jessica Rose — Substack
- X — Nicholas Fabiano on brain-border S1
- X — Jessica Rose (JesslovesMJK)
- X — Annelise Bocquet (@AnneliseBocquet)
- X — Bocquet on SARS-CoV-2 latency/reactivation
- X — Bocquet on spike persistence & immunodeficiency
- X — Bocquet on fatal SARS-CoV-2 reactivation
- X — Genome Defense
- X — SARS-CoV-2 spike (S1) can induce cellular aging in human astrocytes
How to cite
MeasslainteIRL. The Slow Burn: How Persistent Spike Protein, Reactivated Viruses, and Microclots Drive Long-Term Health Issues. Published 20 Oct 2025. Available at: measslainte.com (accessed ).
Ethical Declaration (Purpose & Scope)
This article exists to promote scientific transparency, informed consent, and open discussion on biomedical safety. All data are cited from primary or peer-reviewed sources where available. No medical advice is given.
Contested / Active-Debate Notes
- Microclots: Assay methods and clinical utility are evolving; causality vs correlation remains under study.
- PVS spike detection: LISTEN findings are preprint data; interpretation may change post peer review.
- RAGE/S1 and brain persistence: Human autopsy signals exist; translation to population-level risk needs larger, multi-centre studies.
Quick FAQ
Is "persistence" the same as ongoing infection?
Not necessarily. Studies detect RNA/antigen or protein fragments in tissues/cells; that can reflect residual antigen, low-level replication, or compartmentalized reservoirs. Clinical significance varies by context.
Do vaccines stop brain-border S1 entirely?
Mouse data suggest prior vaccination reduces but does not eliminate S1 accumulation/leak at the skull–meninges–brain interface.
Are microclots unique to COVID?
No—fibrin(ogen) amyloid has been reported in other inflammatory states. What's debated is extent, persistence, and impact in Long COVID/PVS.
What's reasonable for self-care?
General terrain supports (diet quality, oral health, sleep, graded activity) are low-risk adjuncts. Diagnosis/treatment decisions belong with clinicians.
Plain Talk Add-On: The Slow Burn, in real-world language
If science chat makes your eyes glaze over, start here.
The short version (30 seconds):
- Pieces of the virus (or its spike) can hang around longer than expected in some people.
- That can keep the immune system slightly "on," so you feel unwell even after "recovering."
- Old viruses like EBV/HSV can "wake up" when your system is stressed.
- Tiny, stubborn microclots can slow oxygen delivery — think thick sludge in skinny pipes.
- Some folks' brain "filter" (BBB) is leakier by genetics (e.g., APOE4), so inflammation hits harder.
A simple analogy:
- Imagine you spilled glitter in your house. You clean, but weeks later, glitter is still turning up in corners.
- Meanwhile, the pipes have a bit of sludge (microclots), so water pressure (oxygen delivery) isn't great.
- If your air filter (brain barrier) is older or thinner, dust gets through more easily.
How it can feel day to day:
- Brain fog, short breath on stairs, heavy legs, weird heart flutters, sleep that doesn't refresh, headaches after minor effort, and "good days / crash days" that don't follow a pattern.
What this isn't saying:
- Not everyone has this.
- It's not proof of one single cause or a one-size-fits-all cure.
- Research is ongoing; some findings are early or debated.
Practical supports (common-sense, not medical advice):
- Sleep & pacing: Respect your "energy budget" and avoid boom-and-bust cycles.
- Gentle movement: Short walks, light mobility work; increase slowly if tolerated.
- Oral health: Treat gum issues; better mouth health = less background inflammation.
- Food basics: Protein, colorful plants, minimal ultra-processed foods; hydrate.
- Nervous system downshift: Sunlight, breath work, time outdoors, low-stress routines.
- When to get help: New chest pain, severe headaches, one-sided leg swelling, fainting, or stroke-like signs → urgent care. For persistent symptoms, talk to a clinician who takes post-viral issues seriously.
This section is for understanding, not diagnosis. Always work with a clinician for medical decisions.
Microclots & platelet pathology
- Prof. Resia Pretorius — microcirculation & microclots:
- Pretorius & Prof. Doug Kell — latest research and methods:
- Clinical perspective (Laubscher + Pretorius):
Brain/BBB context (APOE4)
- Axel Montagne, PhD — BBB dysfunction & APOE4:
- USC Zilkha Seminar (recording link): APOE4 → BBB breakdown
Brain border / persistence
- Spike at skull–meninges–brain interface (paper walk-through)Why: Ties directly to your brain-border section; quick ref recap.
EBV / latent virus reactivation (mini-series)
- EBV & Long COVID (Part 1 overview)
- Evidence of EBV reactivation in long haul (study walk-through)
BBB & APOE4 (context for vulnerability)
- APOE4 & blood–brain barrier leaks (lay explainer)
Neuro / autoimmunity angle
- Brain autoimmunity in Long COVID (MBP, MOG)
PVS / LISTEN signals
- Yale LISTEN immune signatures in post-vax syndrome (recap)
Clinic-facing overview
- Long-COVID management (broad symptom & systems talk)
Clotting/microclots (mechanisms) — external link
- Spikeopathies: how spike can drive platelet activation & clotting
Watch on React19 →
Disclaimer: Informational only; not medical advice.