Table of Contents
Key Takeaways
- Process 1 vs Process 2 ⚠️: Clinical trial (Process 1) used PCR-amplified DNA template with 44,000 participants; commercial product (Process 2) switched to E. coli-derived plasmid DNA without direct safety comparison
- DNA Contamination: Independent labs across 4 continents confirmed residual plasmid DNA up to 843 ng/dose (84x FDA/WHO 10 ng limit); SV40 promoter sequences detected
- Safety Comparison Removed: Protocol Amendment 20 (Sept 2022) removed Process 1 vs Process 2 safety comparison; only 252 participants received Process 2 during trial (lot EE8493)
- Regulatory Alarm: Health Canada internal emails show "SV40 must be avoided" and urgency to "remedy the situation before Fall 2024 vax campaign"
- Safety Signals: Lymphadenopathy increased 17-fold (0.3% → 5.2%); menorrhagia increased 370-fold (0.065% → 24%); 36% increase in serious adverse events in trial reanalysis
- SV40 Mechanism: January 2024 preprint shows SV40 enhancer functions as somatic hypermutation targeting element—may hijack AID enzyme, impair antibody responses, cause oncogenic mutations
- Manufacturer Knowledge: Moderna's patents explicitly warned DNA fragments "may cause a patient to develop cancer" and "may be incorporated into the genome" years before pandemic rollout
The Unfinished Safety Audit
Imagine you test a prototype car with meticulous care, then send a completely different manufacturing version to dealerships—without telling buyers about the changes. This isn't just about cars; it's what happened with Pfizer's COVID-19 vaccine.
The vaccine used in clinical trials—the one that demonstrated safety and efficacy—was manufactured under "Process 1." The version billions received came from "Process 2." The fundamental question remains: Were they actually the same product?
Key Findings (Expert Summary)
Process 1 used PCR-amplified DNA template with 44,000 participants in C4591001 trial. Process 2 switched to E. coli-derived plasmid DNA for industrial scaling, introducing new contaminants including plasmid DNA and LPS endotoxins. Only 252 participants received Process 2 during the trial (lot EE8493, starting Oct 19, 2020). DNA contamination reached 188-509x FDA/WHO limits (billions of DNA molecules/dose). Safety signals increased dramatically: lymphadenopathy (0.3% → 5.2%), menorrhagia (0.065% → 24%). The Process 1 vs Process 2 safety comparison was removed via Protocol Amendment 20 in Sept 2022.
What This Means (For Everyone)
The COVID vaccine tested on 44,000 people in clinical trials was NOT the same one given to billions worldwide. Pfizer changed their manufacturing method, introducing new ingredients (bacterial DNA and endotoxins) that weren't properly tested. Only 252 people received the new version during the trial, yet this became the global standard without direct safety comparison.
The Bottom Line: When you got your Pfizer vaccine, you likely received a different product than the one proven "safe and effective" in clinical trials.
The Numbers Don't Lie: Process 1 used PCR-amplified DNA template in Phase 3 trial with 44,000 participants. Process 2 switched to E. coli-derived plasmid DNA for commercial scaling. Only 252 vaccine recipients received Process 2 during the trial (lot EE8493 at 4 U.S. sites starting October 19, 2020). Sources: TGA FOI 3659 document 4, FDA batch release data 125742_S1_M5_5351_c4591001-interim-mth6-patient-batches.pdf
A Tale of Two Factories
The Clinical Trial Version (Process 1)
Setting: Laboratory-scale production using PCR-amplified DNA template. Methods: Research-grade purification, small-batch DNA templates. Purpose: Generate data for regulatory approval. Testing: Used in the C4591001 Phase 3 trial (NEJM Protocol, Walsh et al., 2020). Key Feature: No bacterial plasmid DNA or LPS endotoxins from E. coli fermentation.
The Mass Production Version (Process 2)
Setting: Industrial-scale E. coli fermentation for plasmid DNA production. Methods: Revised purification, different enzymes, scaled DNA production. Purpose: Supply global demand (billions of doses). Timeline: Started October 19, 2020; dominated market by late 2021. Contaminants Introduced: Plasmid DNA, lipopolysaccharide (LPS) endotoxins.
The Critical Gap: Pharmaceutical regulations require proof that process changes don't affect product quality or safety (FDA Process Validation Guidelines 1987, WHO Guidelines). This proof was never made public.
The Disappearing Safety Check
Pfizer's original trial design included a crucial safeguard: a cohort of participants would receive both Process 1 and Process 2 material for direct comparison. This wasn't an optional extra—it was fundamental science.
Then, in September 2022, Protocol Amendment 20 removed this objective (Archive of Protocol C4591001 Amendment 20). The planned safety comparison vanished just as Process 2 dominated global supply.
Regulatory Silence on Equivalence: When researchers filed Freedom of Information requests asking how equivalence was demonstrated, regulators acknowledged the question but provided no analytical data. MHRA FOI 23/510 confirmed Process 2 dosing started October 19, 2020, but comparison was abandoned. MHRA response stated "We do not hold the information" when asked for comparability data. TGA FOI 3659 lists Process 1 and Process 2 batches but provides no comparative safety analysis.
The Process 2 Trial Cohort: Only 252 participants received Process 2 during the trial (from lot EE8493). Randomization numbers: 400002–401509 (verified via GitHub R scripts: OpenVaet/pfizer_docs_R). Location: Four U.S. sites starting October 19, 2020.
The DNA Contamination Mystery: From Theory to Regulatory Crisis
The Safety Standard
International guidelines set a clear limit: no more than 10 nanograms of residual DNA per injection (WHO TRS 978, FDA/EMA guidelines). This isn't arbitrary—foreign DNA can trigger immune reactions or, in theory, integrate into human cells.
The Global Laboratory Consensus
Multiple independent laboratories across North America, Europe, and Asia have now confirmed DNA contamination in Process 2 vaccine lots:
Quantified Contamination Levels: Kevin McKernan et al. (2023) found 10-843 ng/dose with SV40 promoter confirmation (OSF Preprint, DOI: 10.31219/osf.io/b9t7m). David Speicher et al. (2024) analyzed 27+ vials showing 0.22-510 ng/dose range (Preprint - Archived, DOI: 10.20944/preprints202408.0331.v1). Phillip Buckhaults (USA) confirmed 1-6 ng/dose in Senate testimony. König/Kirchner (Germany) reported 3,600-5,340 ng/dose (500x regulatory limit).
Contamination Exceeds Limits by 188-509x in multiple independent analyses (billions of DNA molecules per dose).
Why This Matters: Jessica Rose's analysis demonstrates that RNA/DNA hybrids resist enzymatic digestion, extending the biological half-life of contaminants. This means the DNA fragments detected in these vials may remain active in the body far longer than standard degradation models predict.
Reproducible Analysis: GitHub scripts available at OpenVaet/pfizer_docs_R. Scripts include model_process_2_aes.R (Poisson regression showing 264% increase in AEs for Process 2 recipients ≤55 years) and inspect_menstrual_disorders_aes.R (menstrual disorder analysis).
Health Canada's Internal Alarm: The Regulatory Smoking Gun
Internal emails obtained through investigative work by Scoops McGoo reveal a regulatory crisis directly connected to Process 2 manufacturing:
The Unplanned Discovery
Pfizer's Admission: "Yes, because Pfizer did not identify the presence of SV40 promoter enhancer on the plasmid template used to produce mRNA" ( ). Process 2 Connection: This admission directly implicates the commercial manufacturing process where quality controls failed.
). Process 2 Connection: This admission directly implicates the commercial manufacturing process where quality controls failed.
Regulatory Alarm Bells
"SV40 must be avoided!" ( ) - Clear safety principle violation. "Remedy the situation before Fall 2024 vax campaign" (
) - Clear safety principle violation. "Remedy the situation before Fall 2024 vax campaign" ( ) - Urgent timeline for Process 2 resolution. "They do not seem to care much at this moment" (
) - Urgent timeline for Process 2 resolution. "They do not seem to care much at this moment" ( ) - Frustration with Pfizer's response.
) - Frustration with Pfizer's response.
Scientific Concern
"Fragment size is related to the probability of integration" ( ) - fragment size is related to the probability of integration with the human genome. "References: None" beside "minimal safety risk" claim (
) - fragment size is related to the probability of integration with the human genome. "References: None" beside "minimal safety risk" claim ( ) - Unsubstantiated safety assurance minimal safety risk … "References: None".
) - Unsubstantiated safety assurance minimal safety risk … "References: None".
This regulatory crisis context makes the removal of the Process 1 vs Process 2 safety comparison even more concerning.
For detailed laboratory findings on DNA contamination, see SV40 DNA Signals in COVID-19 mRNA Vaccine Vials: What These Independent Labs Reported
The SV40 Enigma: From Cancer Risk to Immune System Threat
Pfizer's manufacturing plasmid included SV40 sequences—fragments from a virus once linked to cancer in early polio vaccines. The company says they're "inert" (Pfizer FAQ), but emerging research reveals a more immediate concern than theoretical cancer risk.
A January 2024 preprint demonstrates that the SV40 enhancer functions as a somatic hypermutation (SHM) targeting element - meaning it can directly interfere with the fundamental process our immune system uses to create effective antibodies (bioRxiv Preprint).
What This Means for Immune Function:
Somatic hypermutation is the process where B-cells "fine-tune" antibodies to better recognize pathogens. AID enzyme is the "master catalyst" that makes this possible. SV40 enhancer can hijack this process, potentially causing:
- Impaired Antibody Responses: Disruption of normal SHM could lead to reduced ability to fight infections
- Oncogenic Mutations: Aberrant targeting of AID could introduce cancer-causing mutations
- Autoimmune Potential: Misdirected hypermutation might generate antibodies that attack the body's own tissues
As immunologist Jessica Rose notes in her analysis of this mechanism: "If we have impaired antibody responses, we have a broken immune system."
This represents a potential immune system threat that was never assessed in the Process 1 vs Process 2 comparability that was never conducted.
For comprehensive analysis of biological mechanisms, see The Stability Trap: How mRNA Vaccine Engineering and DNA Contamination Created a Perfect Storm
Manufacturer Prior Knowledge: Moderna's Patent Admissions
Manufacturers had long recognized the theoretical risks of residual DNA in mRNA production. Moderna's patent filings explicitly discuss:
"DNA fragments resulting from the mRNA production process need to be removed". "Because the remaining DNA fragments may cause a patient to develop cancer". "qPCR can't measure small DNA fragments, yet we have to use another method to quantify them". "Some of the introduced DNA may be incorporated into the genome of the cell and inherited by the offspring".
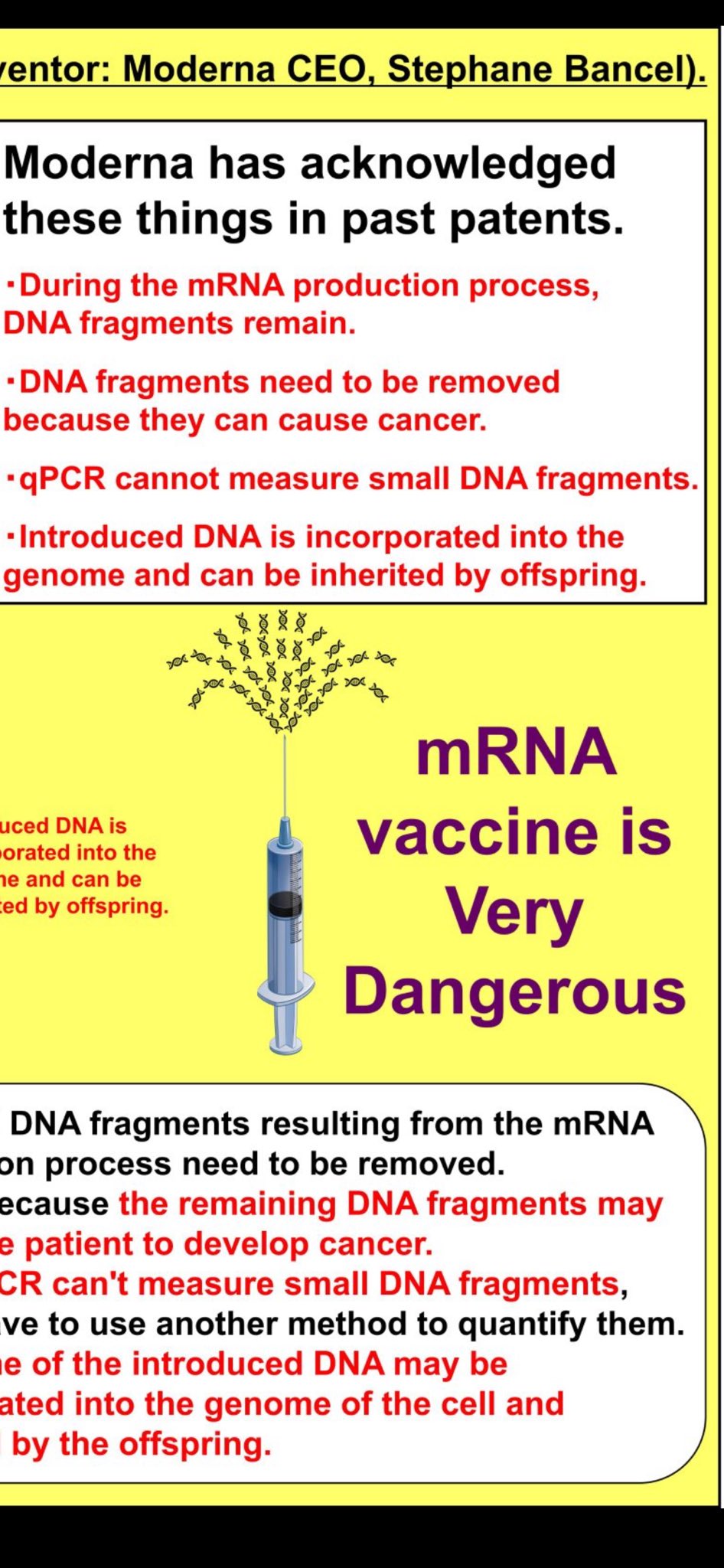
These admissions show the risks were well-understood years before the pandemic rollout, making the discovery of these same contaminants in Process 2 vaccine lots particularly alarming.
The Manufacturing Knowledge Gap
The discovery that manufacturers understood DNA contamination risks years before the pandemic raises critical questions about Process 2 quality control:
Moderna's Patent Warnings Included:
"DNA fragments... may cause a patient to develop cancer". "Introduced DNA may be incorporated into the genome... inherited by offspring". Explicit acknowledgment that qPCR cannot adequately measure small DNA fragments.

Given this prior knowledge, the failure to implement adequate DNA contamination controls in Process 2 manufacturing—and the subsequent removal of Process 1 vs Process 2 safety comparisons—represents a significant breakdown in pharmaceutical quality assurance.
For complete reference documentation, see DNA Contamination & mRNA Vaccine Biology: Curated Reference Roadmap
Safety Signals: Coincidence or Consequence?
The Trial Data Reanalysis
When Dr. Joseph Fraiman re-analyzed the original Pfizer and Moderna trials, he found a 36% increase in serious adverse events in vaccine recipients versus placebo (Vaccine 2022). These weren't sore arms—they were events serious enough to require medical intervention.
Specific Safety Signal Changes: Process 1 vs Process 2
Lymphadenopathy Increase: Process 1 (Trial): 0.3% rate (MHRA Public Assessment Report 2021). Process 2 (Post-marketing boosters): 5.2% rate ([MHRA June 2023 update]). Increase: 17-fold higher than trial baseline.
Menorrhagia (Heavy Menstrual Bleeding):
Process 1 (Trial): 0.065% prevalence (analysis via inspect_menstrual_disorders_aes.R script). Process 2 (Real-world): 24% pooled prevalence (95% CI: 12.8–35.6%) in meta-analysis (Al Kadri et al., PMID: 38199871). Increase: 370-fold higher than trial baseline.
The Biological Evidence
Spike Protein Detection: Dr. Lael Yonker found circulating spike protein in teens with post-vaccine myocarditis (Circulation 2023). Spike Persistence: Multiple studies detected spike protein lingering in tissues months after vaccination. DNA Inflammation Pathways: Research confirms foreign DNA can activate inflammatory responses through cGAS-STING pathways (Front Immunol 2023). LPS Endotoxin Effects: Process 2 E. coli fermentation introduces lipopolysaccharide that can trigger neuroinflammation via TLR4 activation or TNFα/TNFR2 pathways.
Counterarguments and Limitations
Regulatory Bridging Studies
Regulators assert that Process 1 and Process 2 are equivalent under 1987 FDA guidelines stating "the process is the product" (TGA comparability report - Archived). However, critics note missing comparisons as MHRA FOI confirms no direct safety comparison was conducted. While traditional bridging is standard practice for biological products, Process 2 introduced fundamentally new contaminants (plasmid DNA, LPS). The rapid timeline and emergency pandemic context may have accelerated acceptance without full characterization.
Fact-Check Claims
Some organizations claim that post-EUA testing expanded to thousands of participants (TechARP fact-check). However, Process 2 exposure was limited to 252 participants during controlled trial period. Post-marketing data includes no controlled comparisons against Process 1 baseline. Safety surveillance through passive reporting systems lacks control groups for process-specific analysis.
Limitations of Current Evidence
Preprint Status: Several key contamination analyses remain in preprint form (McKernan et al., Speicher et al.), though methodology is transparent and reproducible.
Confounding Factors: Real-world safety signal increases could be influenced by different patient populations (elderly, immunocompromised), multiple vaccine exposures (boosters, mixed schedules), background COVID-19 infection effects, and enhanced surveillance and reporting awareness.
Dose-Response Relationships: Clear causal links between specific contamination levels and adverse outcomes require further study, though biological mechanisms are established.
Traffic-Light Snapshot: Claims vs Reality
"The manufacturing change was rigorously validated"
Company Claim: Process 2 equivalent to Process 1. Evidence: Comparison cohort removed from trial; FOI requests yield no data; SV40 sequence was unexpected finding; Multiple independent labs find DNA contamination in Process 2 vials. Verdict: ❌ Validation failure - No public proof despite global lab confirmations.
"DNA levels are within safe limits"
Regulatory Standard: 10 ng/dose maximum. Independent Findings: Up to 843 ng/dose reported (84x limit); SV40 sequences present; Health Canada emails show internal alarm; SV40 disrupts somatic hypermutation. Verdict: ❌ Unacceptable risk - mechanism identified for immune system harm.
"Safety signals are coincidental"
Dismissal: Billions administered safely. Evidence: 36% serious adverse event increase in trials; biological mechanisms identified; Moderna patents acknowledge cancer risk from DNA fragments. Verdict: ❌ Requires proper investigation, not dismissal.
The Human Cost of Technical Decisions
Behind the manufacturing specifications and quality control documents are real people: the trial participants who volunteered for what they believed was rigorous science; the billions who received injections based on trial data of a different product; the doctors seeing unexplained inflammatory conditions in previously healthy patients; the families who trusted that "safe and effective" meant the product was thoroughly characterized.
What Transparency Would Look Like
- Release the Data: Publish all comparability studies submitted to regulators
- Independent Testing: Multi-laboratory analysis of retained Process 1 vs Process 2 vials
- Clinical Correlation: Examine adverse event rates by manufacturing lot
- Full Disclosure: Document all process changes and their justifications
Related Investigations
For readers seeking deeper analysis of specific aspects:
The Stability Trap: How mRNA Vaccine Engineering and DNA Contamination Created a Perfect Storm - Analysis of how mRNA stability modifications intersect with DNA contamination to create biological risks.
SV40 DNA Signals in COVID-19 mRNA Vaccine Vials - Comprehensive laboratory findings from 9+ independent research teams confirming DNA contamination.
DNA Contamination & mRNA Vaccine Biology: Curated Reference Roadmap - Complete reference database with primary sources and regulatory documents.
The Core Question
When you received your Pfizer COVID-19 vaccine, did you get the carefully tested clinical trial version—or something fundamentally different that was never directly compared for safety?
The discovery of unexpected DNA contaminants that caused significant internal alarm at Health Canada, combined with manufacturers' own prior knowledge of these risks, suggests the answer is not reassuring. The fact that these fundamental questions remain unanswered represents a failure of scientific transparency at the highest levels.
The removal of Process 1 vs Process 2 safety comparisons, combined with the subsequent discovery of DNA contamination that regulators were privately alarmed about, suggests we may never know what differences existed between the vaccine that was tested and the vaccine that was delivered to billions.
see the very latest discussions over on substack Dr Jessica Rose (https://substack.com/home/post/p-175987412) Kevin McKernan (https://anandamide.substack.com/p/pfizers-failure-to-linearize-the) and Dr David Speicher (https://substack.com/@drdavidspeicher/note/p-159309301?utm_source=notes-share-action&r=15i0bd) follow them all on X support their work on substack these guys are literally the tip of the spear.
Comprehensive Source Library
Scientific Papers & Preprints
NEJM Protocol (Walsh et al., 2020): Supplementary protocol for BNT162b2 Phase 3 trial C4591001. Link
McKernan et al. (2023): DNA contamination analysis with SV40 sequences. OSF Preprint, DOI: 10.31219/osf.io/b9t7m
Speicher et al. (2024): Residual plasmid DNA quantification in 27+ vials. Preprint - Archived, DOI: 10.20944/preprints202408.0331.v1
Al Kadri et al. (2023): Meta-analysis of menorrhagia prevalence (PMID: 38199871). PMC
Levi et al. (2024, BMJ): Effect of mRNA vaccine manufacturing processes on efficacy/safety. BMJ
Fraiman et al. (2022, Vaccine): Reanalysis of serious adverse events in mRNA trials. PubMed
Regulatory Documents
FDA Process Validation Guidelines (1987): General principles for biopharmaceutical manufacturing. FDA
TGA FOI 3659 Document 4: BNT162b2 Comparability Report listing Process 1/2 batches. TGA - Archived
FDA Patient Batches Document: C4591001-interim-mth6-patient-batches.pdf tracking lot shipments. PHMPT
MHRA FOI 23/510: Confirmation of Process 2 dosing and abandoned comparison. WhatDoTheyKnow
MHRA Public Assessment Report (2021): Lymphadenopathy rates from trial. UK Gov
WHO TRS 978: DNA contamination limits for biological products. WHO
Open Source Analysis
OpenVaet GitHub Repository: Reproducible R scripts for Process 2 analysis. GitHub
Scripts include model_process_2_aes.R for Poisson regression showing 264% AE increase, inspect_menstrual_disorders_aes.R for menstrual disorder analysis, and Process 2 subject identification via randomization numbers 400002–401509.
Media Coverage
Dr. Paul Offit Video: "The process is the product" explanation (39s). Twitter
Bret Weinstein Video: mRNA switch analysis (5m). Twitter
Dr. Ryan Cole Video: Process 1 vs 2 overview (1m40s). Twitter
Counterarguments & Fact-Checks
TechARP Fact-Check (2025): Claims of expanded post-EUA testing. TechARP
Health Canada Emails: Internal regulatory communications about SV40 discovery. Scoops McGoo
This investigation exists because independent scientists refused to accept that manufacturing changes don't require rigorous validation. In a properly functioning regulatory system, these questions would have been answered before products reached the public.