Table of Contents
Key Takeaways
- Neurospirochetosis Evidence 🧠: Autopsy series from 1911 to present describe living Borrelia burgdorferi spirochetes in brains of deceased MS patients across at least 10 countries
- Testing Limitations: Standard Lyme testing plagued by false negatives due to strain variability, immune response differences, and rigid diagnostic criteria; "negative" result does not rule out infection
- Beyond Ticks: Transmission may extend beyond ticks to other arthropods (fleas, mites, lice, bed bugs) and bodily fluids (semen, blood, urine, saliva); sexual/contact transmission biologically plausible
- Alzheimer's Connection: Borrelia burgdorferi found in ~25% of Alzheimer's cases (far more than controls); periodontal Treponemas observed in >90% of AD brains
- Inflammatory Food Triggers: Gluten (zonulin → leaky gut), A1 dairy (BCM-7 → gut/brain inflammation), legumes (lectins/phytates), nightshades, and industrial seed oils feed inflammation
- Antimutagenic Support: Cruciferous vegetables (sulforaphane), green tea (EGCG), red grapes/blueberries (resveratrol), onions/capers (quercetin), and fiber provide genome defense
- Family Clustering: Likely due to shared exposure (same house, pests, pets, water) and genetic propensity affecting ability to clear infections
Start here
If you look, you find it. For over a century, pathologists have reported spirochetes in brains — then we pretended not to see them. Lyme's agent, Borrelia burgdorferi, doesn't stop at joints; it crosses endothelium, enters the CNS, and leaves footprints that overlap MS. Tests miss it. Gatekeepers dismiss it. Patients live it.
What this gives you:
- What autopsies and stains actually show about Borrelia in brains
- Why "negative" tests don't mean nothing's there
- How transmission likely extends beyond ticks
- What you can do now: remove food triggers, stack antimutagens, and track your response
Neurospirochetosis & MS
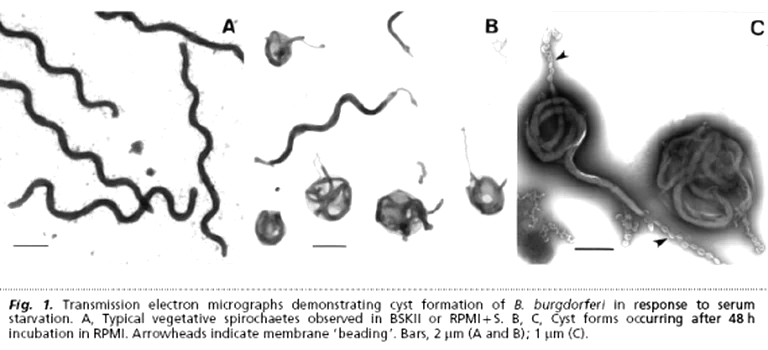
MS may be linked to spirochetes — specifically Borrelia burgdorferi sensu lato, the bacterium behind Lyme disease. Across at least ten countries and more than a century of reports, autopsy series (1911 → present) describe living Lyme spirochetes in the brains of deceased MS patients. Meanwhile, standard testing in living patients is plagued by false negatives — and many still test positive for active Lyme.
Receipts that keep getting erased:
- Living Borrelia detected in MS brains.
- Borrelia burgdorferi found in ~25% of Alzheimer's cases, far more than in controls.
- Periodontal Treponemas observed in >90% of Alzheimer's brains.
The thread through it all: neurospirochetosis is visible when you actually look.
Evidence for a Conspiracy of Silence
You don't have to take my word for it. Pathologist Dr. Alan B. MacDonald:
"Microscopic images of cystic spirochetes are difficult to ignore… endowments have nearly expunged all cystic spirochetal image data from current textbooks… Variously sized cystic spirochetal profiles within diseased nerve cells explain: Lewy bodies, Pick bodies, ALS spherical bodies, Alzheimer plaques… spirochetes are hiding in plain sight."
https://doi.org/10.1016/j.mehy.2006.02.035
Lyme vs MS — Tom Grier's Challenge
Microbiologist Tom Grier doesn't mince words: MS isn't a standalone disease; Lyme can drive the syndrome. His own case shows why the fight is personal — and why the establishment plugs its ears.
Highlights:
- Mother-to-child transmission of Borrelia across the womb
- B. burgdorferi + B. miyamotoi with amyloid plaques in AD brains
- Borrelia in Lewy body dementia
- Nematode worms reported in CSF of MS patients
- Borrelia signals in glioblastoma multiforme
- B. mayonii and B. burgdorferi found in human testicles
- Borreliosis is a family, not one bug/one symptom — B. miyamotoi sits alongside B. burgdorferi in brains → eradicate together
Download: Tom Grier — MS and Lyme (PDF)
Testing: why "negative" isn't negative
Lyme testing misses people.
- Strain variability: narrow antigen panels ignore the rest.
- Immune variability: not everyone mounts textbook antibodies.
- Gatekeeping: rigid criteria write patients off while labs/insurers save face.
Result: misdiagnosis, delay, damage — while the infection marches on.
Transmission: the map is bigger than ticks
Ticks (Ixodes spp.) are the primary vector — but the story doesn't end there.
- Other arthropods: bed bugs, fleas, mites, lice have been implicated to varying degrees.
- Bodily fluids: Borrelia DNA/organisms detected in semen, blood, urine, saliva — yes, that puts sexual/contact transmission on the table.
Why it clusters in families
- Genetic propensity: some people struggle to clear infections.
- Shared exposure: same house, same pests, same pets, same water — same risk.
Human-to-human?
Lyme is treatable with antibiotics, and there's no sign of casual person-to-person spread during treatment.
For people living with Lyme: calm the fire first (food → inflammation)
A deeper dive into the biochemical triggers of inflammation — and the foods that silently feed the fire — informed by Wahls-style protocols and gut–immune research. Whether you're dealing with autoimmunity, neuroinflammation, chronic fatigue, IBD, MS, arthritis, or post-viral syndromes, removing inflammatory inputs is a power move.
🚫 1) Gluten-containing grains
Gluten → zonulin → leaky gut; LPS translocation; molecular mimicry.
🥛 2) Dairy (esp. conventional A1 dairy)
A1 casein → BCM-7 (gut/brain inflammation); lactase deficits; casein antibodies.
✅ Later (if tolerated): A2 or fermented goat/sheep — exclude in elimination.
🌱 3) Legumes
Lectins bind epithelium; phytates steal minerals; soy adds glyphosate/phytoestrogens; peanuts carry aflatoxins.
⚠️ Pressure-cooking helps, doesn't erase all anti-nutrients — avoid early.
🍅 4) Nightshades (if sensitive)
Alkaloids (solanine, capsaicin) can irritate gut, activate mast cells, and trigger neurogenic inflammation.
🍬 5) Processed sugars & refined carbs
Glucose spikes → insulin surges → NF-κB/IL-6; dysbiosis; AGEs harm mitochondria, collagen, neurons.
🌽 6) Industrial seed oils
High linoleic acid (ω-6) → pro-inflammatory eicosanoids; easy oxidation → lipid peroxides.
✅ Swap: extra virgin olive oil, coconut oil, ghee, avocado oil, animal fats.
🧈 7) Trans fats
Wreck membranes, insulin signaling, mitochondria.
🚫 Zero is the target.
🧪 8) Artificial additives & preservatives
Mast-cell activation, histamine load; excitotoxins (MSG/aspartame) can worsen neuroinflammation; preservatives distort microbiota.
🍏 9) FODMAPs (for sensitive guts)
Fermentable carbs can fuel IBS/SIBO; raise permeability and mast-cell activity.
🧠 Low-FODMAP is a tool, not a lifestyle — use briefly to calm the gut.
Simple swaps you can live with
| Swap this | For this |
|---|---|
| Wheat bread/pastries | Buckwheat, cassava, gluten-free sourdough |
| A1 cow dairy | A2/fermented goat or sheep (after elimination) |
| Seed oils (soy/corn/canola) | Extra virgin olive oil, ghee, avocado oil |
| Refined sugar/HFCS | Berries, 85% dark chocolate, raw honey (sparingly) |
| Nightshades (if sensitive) | Squash, beets, carrots, cucumber |
Polyphenol targets (food first)
| Food | Key compound | Why it's here |
|---|---|---|
| Green tea | EGCG | Angiogenesis + NF-κB modulation |
| Red grapes/blueberries | Resveratrol | EMT/STAT3 control, apoptosis |
| Onions/capers | Quercetin | CSC inhibition, PI3K/Akt |
| Parsley/celery/chamomile | Apigenin | mTOR↓, pro-autophagy |
| Broccoli sprouts | Sulforaphane | Phase II detox (GST), redox support |
30–60 day reset (save this)
- Eliminate: gluten, A1 dairy, legumes, seed oils, sugar, additives (trial nightshades/FODMAPs if symptomatic).
- Add: crucifers/sprouts, green tea, berries, EVOO, spices (turmeric).
- Track daily: sleep, pain (0–10), neuro sx, GI/stools, energy, HR/palps, foods.
- Reintroduce: one item every 3–4 days; note changes at 24/48/72 h.
Genome defense: antimutagens that matter
High-heat meat makes heterocyclic aromatic amines (HAAs) — genotoxic. Your counter-punch: crucifers (sulforaphane → GST), curcumin, resveratrol, chlorophyllin/green-tea polyphenols, and fiber to bind/excrete mutagens. That combo down-modulates CYP450 activation, shifts Phase II detox, controls ROS, and tunes gene expression.
- Review of 160+ studies on antimutagenic strategies: https://doi.org/10.1080/10408440091159176
Erich Traub, LCMV & the biowarfare shadow
You want the uncomfortable part? Here it is.
- Erich Traub worked on stealth biological agents designed to evade detection and cause chronic disease.
- LCMV is neurotropic and often misdiagnosed — a template for immune-evasive persistence.
- Lyme as a weaponized platform: allegations of animal passages to refine organisms and keep plausible deniability — "just nature."
- Ticks are perfect vectors: long feed times, painless attachment, direct bloodstream access, broad host range.
- The line between "research" and deployment is thin — and smudged.
Selected papers & reviews (quick links)
Spirochetes in neurodegeneration / brain tissue
- Miklossy J. Alzheimer's disease — a neurospirochetosis. J Neuroinflammation (2011).
https://doi.org/10.1186/1742-2094-8-90 - Riviere GR, et al. Oral Treponema in human brain associated with AD. J Alzheimers Dis (2002).
https://doi.org/10.3233/jad-2002-4203 - MacDonald AB. Plaques of AD originate from cystic spirochetes. Med Hypotheses (2006).
https://doi.org/10.1016/j.mehy.2006.02.035
Persistence / models
- Embers ME, et al. Borrelia persistence post-antibiotics in macaques. PLoS ONE (2012).
https://doi.org/10.1371/journal.pone.0029914 - Sapi E, et al. Biofilm phenotype of Borrelia burgdorferi. Eur J Microbiol Immunol (2012).
https://doi.org/10.1556/EuJMI.2.2012.4.4
Endothelial mechanics / transmigration
- Niddam AF, et al. Plasma fibronectin stabilizes Borrelia–endothelium catch-bonds under shear. PNAS (2017).
https://doi.org/10.1073/pnas.1615007114
Cardiac involvement
- Yeung C, Baranchuk A. Diagnosis and Treatment of Lyme Carditis. J Am Coll Cardiol (2019).
https://doi.org/10.1016/j.jacc.2018.11.035
Diagnostics
- Branda JA, et al. Two-tiered testing with C6 ELISA + immunoblot. Clin Infect Dis (2010).
https://doi.org/10.1086/648674 - Fallon BA, et al. Repeated IV antibiotics for Lyme encephalopathy — RCT. Neurology (2008).
https://doi.org/10.1212/01.WNL.0000284604.61160.2d
Food → barrier & inflammation
- Fasano A. Zonulin and intestinal barrier. Physiol Rev (2011).
https://doi.org/10.1152/physrev.00003.2008 - A1 dairy → BCM-7
- Kamiński S, et al. β-casein polymorphism & health. J Appl Genet (2007).
https://doi.org/10.1007/BF03195213 - Jinsmaa Y, Yoshikawa M. Enzymatic release of BCM-7. FEBS Lett (1999).
https://doi.org/10.1016/S0014-5793(99)01166-4
- Kamiński S, et al. β-casein polymorphism & health. J Appl Genet (2007).
Lectins / antinutrients
- Pusztai A, Bardocz S. Plant Lectins. Taylor & Francis (1996).
https://doi.org/10.1201/9781482295202 - Liener IE. Antinutritional soybean components. Crit Rev Food Sci Nutr (1994).
https://doi.org/10.1080/10408399409527653
Aflatoxins
- IARC Monographs — Aflatoxins. https://publications.iarc.fr/
Seed oils / linoleic acid
- Ramsden CE, et al. Dietary LA and CHD outcomes. BMJ (2013).
https://doi.org/10.1136/bmj.e8707 - Kaur N, et al. Linoleic acid & inflammation. BBA (2014).
https://doi.org/10.1016/j.bbalip.2014.04.001
Sugar → NF-κB / AGEs
- Brownlee M. The pathobiology of AGEs. Physiol Rev (2001).
https://doi.org/10.1152/physrev.2001.81.1.1 - Devaraj S, et al. Glucose induces NF-κB activation. AJCN (2002).
https://doi.org/10.1093/ajcn/75.1.2
Low-FODMAP (IBS tool)
- Halmos EP, et al. Low-FODMAP reduces IBS symptoms. Gastroenterology (2014).
https://doi.org/10.1053/j.gastro.2013.09.046
Antimutagenic nutrition
- HAA antimutagen review (160+ studies).
https://doi.org/10.1080/10408440091159176 - Polyphenols vs pancreatic cancer (pathways & CSCs).
https://doi.org/10.3390/antiox9080651 - Diet & colorectal cancer (microbiome, epigenetics).
https://doi.org/10.3390/nu13010143 - Annual Review of Nutrition (2008) — Nutrition & Mutagenesis.
https://doi.org/10.1146/annurev.nutr.28.061807.155449
Bottom line
Borrelia in the brain isn't rare — it's ignored. Strip out what feeds inflammation, feed what protects your genome, and let your body show you what the committees won't.
Educational content, not medical advice. Work with a clinician for diagnosis/treatment.