Table of Contents
Key Takeaways
- DNA Contamination Confirmed ⚠️: Multiple independent labs (9+ teams across four continents) have documented residual plasmid DNA in mRNA vaccine vials, with levels exceeding regulatory limits by up to 627-fold
- Biologically Active Contaminants: Sequencing reveals bacterial m⁶A methylation patterns and unmethylated SV40 promoters—these are not inert fragments but functionally active genetic elements
- Somatic Hypermutation Risk: SV40 enhancer may hijack AID enzyme, potentially redirecting antibody diversification and causing impaired immune responses or oncogenic mutations
- Regulatory Transparency Gap: Internal Health Canada emails show alarm over SV40 contamination while public assurances maintained safety—regulatory fracture documented
- Process Change Without Validation: Pfizer's clinical trial (Process 1) used PCR-amplified DNA; commercial product (Process 2) switched to E. coli plasmid DNA without direct safety comparison
- Manufacturer Prior Knowledge: Moderna's patents explicitly warned DNA fragments "may cause a patient to develop cancer" and "may be incorporated into the genome" years before pandemic rollout
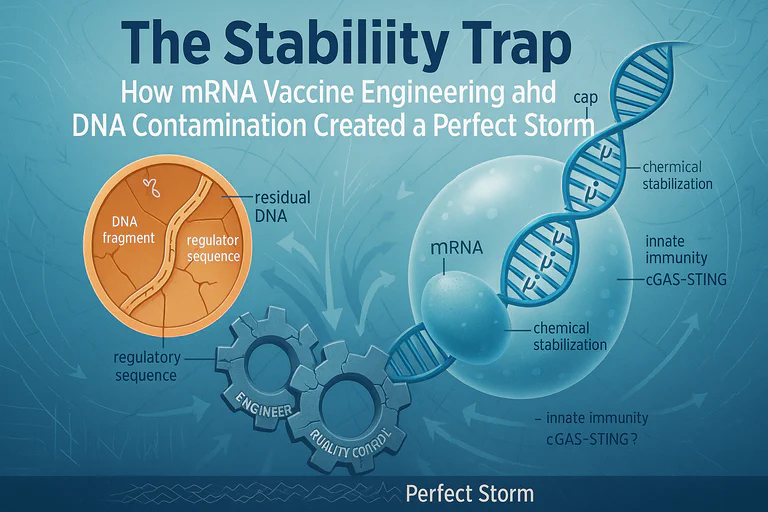
The Engineering Triumph & Its Unintended Biological Price
Drew Weissman and Katalin Karikó won a Nobel Prize for solving a fundamental problem: making mRNA stable enough to work as a drug. The m1Ψ (pseudouridine) modification was a masterstroke—it made synthetic mRNA "invisible" to our innate immune system. Lipid Nanoparticles (LNPs) provided the armored delivery system.
But in medicine, every solution begets new questions. The very modifications that stopped our bodies from breaking down synthetic mRNA may have created a different, unanticipated reality: a vaccine that doesn't clear on schedule, leading to prolonged spike protein production and a cascade of biological consequences we were never prepared for.
The Manufacturing Change Nobody Discussed: Process 1 vs Process 2
The Unannounced Transition
The vaccine used in clinical trials—the one that demonstrated safety and efficacy—was manufactured under "Process 1." The version billions received came from "Process 2." The fundamental question remains: Were they actually the same product?
The Disappearing Safety Check
Pfizer's original trial design included a crucial safeguard: a cohort of participants would receive both Process 1 and Process 2 material for direct comparison. This wasn't an optional extra—it was fundamental science.
Then, in September 2022, Protocol Amendment 20 removed this objective. The planned safety comparison vanished just as Process 2 dominated global supply.
When researchers filed Freedom of Information requests asking how equivalence was demonstrated, regulators acknowledged the question but provided no analytical data (MHRA FOI 23/510).
For detailed analysis of manufacturing changes and regulatory transparency gaps, see Pfizer Process 1 vs Process 2: What Changed, What's Measured, and What Remains Uncertain
The Contamination Problem: It's Not Just DNA, It's Biologically Active DNA
Multiple independent labs across four continents have found significant levels of plasmid DNA fragments in vaccine vials. But new forensic evidence reveals this isn't just about quantity—it's about biological activity.
The Global Laboratory Consensus
Independent Lab Confirmations (9+ teams):
- McKernan (USA) – Found residual plasmid DNA in commercial vials at levels far exceeding standard thresholds (10-843 ng/dose)
- Buckhaults (USA) – Cancer geneticist confirmed plasmid DNA contamination and oncogenic risk in Senate testimony
- Speicher et al. (International) – Comprehensive analysis of 27 mRNA vaccine lots found dsDNA contamination in 100% of samples; DOI independently verified via resolver but not directly on publisher site
- König/Kirchner (Germany) – Reported 3,600-5,340 ng/dose (500x over limit)
- Kämmerer (Germany) – Identified SV40 promoter/enhancer and LNP-mediated delivery
- Raoult (France) – Found full plasmid recovery in vials
- Wang/Kim (USA) – High-school replication project confirming plasmid detection
- Haley (USA) – Independent confirmation of SV40 promoter presence
- Anonymity (Europe) – Multiple independent European labs confirming contamination (some choosing anonymity)
Quantified Oncogenic Risk: The Speicher et al. Peer-Reviewed Data
A 2025 peer-reviewed study by Speicher, Rose, McKernan et al. provided the first comprehensive quantification of residual plasmid DNA and SV40 sequences in commercial vaccine vials:
Key findings:
- Residual DNA exceeded EMA/FDA limits by up to 627-fold
- SV40 promoter-enhancer detected at 0.25–23.72 ng/dose in Pfizer vials
- Oxford Nanopore sequencing revealed fragments up to 3.4 kb — large enough for functional integration
- 815-fold variance in dsDNA levels between batches, indicating severe quality control failures
The magnitude of exceedance and batch variability represents not just a quantitative issue, but evidence of systemic manufacturing control failure.
(Speicher et al., 2025, DOI: 10.1080/08916934.2025.2551517) ⚠️ Note: DOI resolves via DOI resolver and is listed in third-party indexes, but direct verification on the publisher's official platform was not possible at time of writing.
The New Forensic Evidence: A "Smoking Gun"
Sequencing of Pfizer lot FL8095 reveals the contamination is far more dangerous than previously understood (McKernan et al., 2025).
Key Findings:
- Bacterial Methylation Signature: The DNA shows N⁶-methyladenine (m⁶A) methylation in GATC motifs—the unmistakable fingerprint of E. coli "Dam" methylase.
- Incomplete Linearization: Evidence of plasmid fragments that failed to be properly linearized, with potential for circular plasmid forms to persist.
- "Naked" SV40 Enhancer: The SV40 promoter/enhancer region was notably unmethylated, meaning it's fully active.
The Bottom Line: We are not dealing with generic "DNA fragments." We are dealing with specifically modified, immunostimulatory, bacterial plasmid DNA with active viral promoters, delivered directly into human cells via LNPs.
🔗 https://www.zenodo.org/records/17281691
For comprehensive laboratory findings and global scientific consensus, see SV40 DNA Signals in COVID-19 mRNA Vaccine Vials: What Three Independent Labs Reported
The Regulatory Crisis: Health Canada's Internal Alarm
Internal emails obtained through investigative work by Scoops McGoo reveal a regulatory crisis concealed from public view:
The Unplanned Discovery
- Pfizer's Admission: "Yes, because Pfizer did not identify the presence of SV40 promoter enhancer on the plasmid template used to produce mRNA" (Email Pg. 235)
- Quality Control Failure: Manufacturer's own controls failed to detect SV40 sequence
Regulatory Alarm Bells
- "SV40 must be avoided!" (Pg. 66) - Clear safety principle violation
- "Remedy the situation before Fall 2024 vax campaign" (Pg. 141) - Urgent timeline for resolution
- "They do not seem to care much at this moment" (Pg. 151) - Frustration with Pfizer's response
Scientific Concern
- "Fragment size is related to the probability of integration" (Pg. 205) - Direct genotoxicity discussion
- "References: None" beside "minimal safety risk" claim (Pg. 203) - Unsubstantiated safety assurance
Manufacturer Prior Knowledge: The Moderna Patent Admissions
Modernas's own intellectual property reveals they understood the risks years before COVID-19:
Explicit Warnings in Patent Documentation
- "DNA fragments resulting from the mRNA production process need to be removed"
- "Because the remaining DNA fragments may cause a patient to develop cancer"
- "qPCR can't measure small DNA fragments, yet we have to use another method to quantify them"
- "Some of the introduced DNA may be incorporated into the genome of the cell and inherited by the offspring"
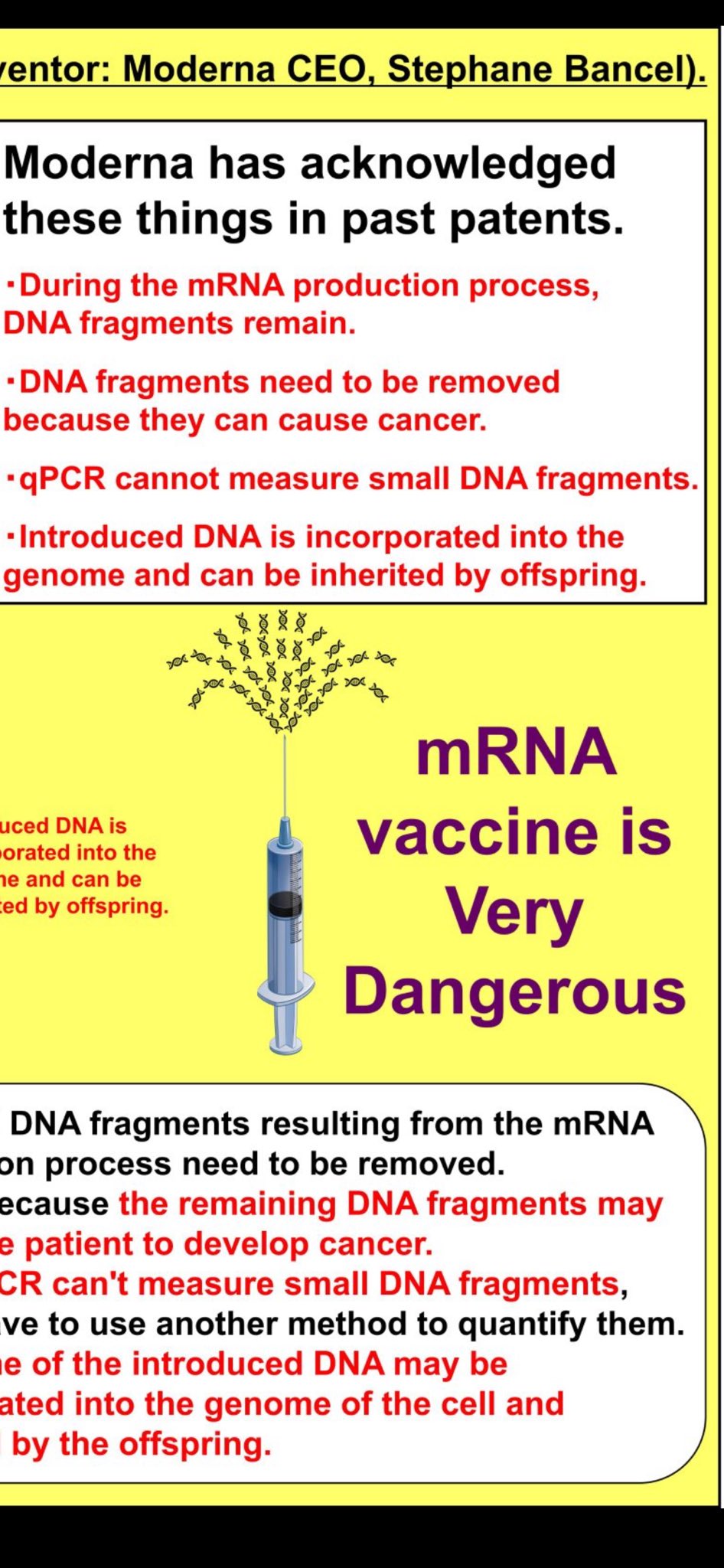
These admissions demonstrate that DNA contamination risks were well-understood, documented, and explicitly warned against in manufacturer's own patent filings years before the pandemic vaccine rollout.
The Biological Mechanisms: Multiple Pathways to Harm
The combination of persistence and biologically active contamination creates several plausible mechanisms for harm.
Mechanism 1: The Innate Immune Cascade
The Chain Reaction:
- Foreign DNA Detection: The m⁶A-methylated bacterial DNA is a potent ligand for innate immune sensors (cGAS-STING and TLR9).
- Chronic Alarm Signal: These sensors trigger pro-inflammatory cascades (Type I Interferons, NF-κB, IL-6, TNF-α).
- Persistence Problem: The stabilized mRNA/LNP platform means this signal isn't transient—it's a chronic, low-level alarm.
- Consequences: Oxidative stress, mitochondrial damage, impaired DNA repair, immune exhaustion, and loss of self-tolerance.
Mechanism 2: The Somatic Hypermutation Sabotage
A January 2024 preprint reveals an even more concerning mechanism: the SV40 enhancer functions as a somatic hypermutation targeting element (bioRxiv).
Understanding the Threat:
- Somatic Hypermutation (SHM): The process where B-cells create antibody diversity by fine-tuning antibodies
- AID Enzyme: The "master catalyst" that enables antibody diversification through targeted mutation
- SV40 Hijacking: Can redirect SHM machinery, potentially causing:
Potential Consequences:
- Impaired Antibody Responses: Disrupted SHM → reduced infection fighting capability
- Oncogenic Mutations: Aberrant AID targeting → cancer-causing DNA changes
- Autoimmune Development: Misdirected hypermutation → self-attacking antibodies
As immunologist Jessica Rose notes: "If we have impaired antibody responses, we have a broken immune system."
Mechanism 3: Genomic Integration Risk
- Reverse Transcription Potential: Aldén et al. found BNT162b2 mRNA can be reverse-transcribed into DNA in human liver cells
- SV40 Promoter Activity: Known to drive high-level gene expression in mammalian cells
- Historical Precedent: SV40's oncogenic potential documented since polio vaccine contamination
Mechanism 4: Nuclear Localization and Insertional Mutagenesis
The SV40 promoter-enhancer sequence functions not just as a transcriptional activator, but as a nuclear localization signal (NLS) — a molecular "passport" that actively transports DNA into the cell nucleus.
The Integration Pathway
- NLS-mediated nuclear entry: The SV40 promoter contains sequences recognized by importin proteins that shuttle cargo through nuclear pores
- Genomic integration: Once in the nucleus, DNA fragments can integrate into chromosomes via:
- Non-homologous end joining (NHEJ) during DNA repair
- Retrotransposon-mediated insertion
- Double-strand break repair mechanisms
- Oncogene activation: If SV40 integrates upstream of proto-oncogenes (e.g., MYC, RAS family), its strong promoter drives constitutive oncogene expression
- Tumor suppressor inhibition: Integration into or near tumor suppressors (e.g., p53, BRCA1) can disrupt their function
Precedent: This mechanism mirrors retroviral oncogenesis, where avian leukosis virus (ALV) insertions upstream of c-MYC caused B-cell lymphomas in chickens by hijacking the gene's expression. (Weinberg, Biology of Cancer, 2014)
Regulatory context: The FDA's Keith Peden established the 10 ng/dose DNA limit specifically to minimize insertional mutagenesis risk in biologics. The 627-fold exceedance in some lots represents a catastrophic regulatory failure.
SV40 classification: The International Agency for Research on Cancer (IARC) classifies SV40 as a Group 2A carcinogen (probably carcinogenic to humans) based on:
- Tumor induction in animal models (hamsters, mice)
- Detection in human tumors (mesotheliomas, brain tumors, osteosarcomas)
- In vitro transformation of human cells
The Evidence of Persistence & Immune Dysregulation
Spike Protein and Component Persistence
Röltgen et al. (2022) - Yale Study – FOUNDATIONAL EVIDENCE: Showed mRNA and spike antigen persist in germinal centers of lymph nodes for at least 60 days post-vaccination. 🔗 https://www.cell.com/cell/fulltext/S0092-8674(22)00076-9
Patterson et al. (2021) – Found S1 sub-units of the spike protein in monocytes for up to 15 months. 🔗 https://www.frontiersin.org/articles/10.3389/fimmu.2021.746021/full
Speicher et al. (2025) – CRITICAL UPDATE: Comprehensive analysis found direct correlation between higher DNA levels and increased frequency of severe adverse events reported to VAERS. DOI: 10.1080/08916934.2025.2551517 (See main citation above)
Epidemiological Signals: Temporal Association with Cancer Mortality
Japan excess cancer mortality (2022): Ueda et al. (2024) analyzed age-adjusted cancer mortality in Japan following widespread mRNA vaccination:
- 7,162 excess cancer deaths in 2022 (95% CI: 4,786–9,522)
- Increase observed after the third mRNA-LNP dose (booster campaign)
- Statistically significant deviation from pre-pandemic and early-pandemic trends
- Age-adjusted to control for demographic shifts
Important caveats:
- Temporal association does not prove causation
- Multiple confounders exist (delayed screenings, pandemic stress, healthcare disruption)
- Japan had high vaccination rates with mRNA vaccines (Pfizer/Moderna dominant)
- Signal requires replication in other high-vaccination countries with robust registries
(Ueda et al., 2024, PMC11077472) ⚠️ RETRACTED: This article has been retracted by the publisher. An expression of concern was also published. The findings are no longer considered reliable scientific evidence.
VAERS cancer reporting: Elevated proportional reporting ratios (PRR) for various cancers post-mRNA vaccination have been documented in pharmacovigilance databases, though underreporting (estimated 1–10% capture rate) and reporting bias complicate interpretation.
Conflicting signals: U.S. cancer registry data (Siegel et al., 2023) showed no significant increase in overall cancer incidence during 2021–2022, though lag time for solid tumor detection (typically 5–10 years) means early signals may not yet be visible in population-level data. (Cancer Statistics, 2023)
The Perfect Storm: How All Elements Converge
The Intersecting Risk Factors:
Engineering Persistence Platform
- Stabilized mRNA (m1Ψ modification)
- Efficient LNP delivery system
- Prolonged spike protein production
Biologically Active Contamination
- Bacterial methylated DNA (m⁶A signatures)
- Active SV40 viral promoters
- Plasmid DNA with integration potential
Immune System Interference
- Chronic innate immune activation (cGAS-STING/TLR9)
- Somatic hypermutation disruption (AID targeting)
- Antibody diversity compromise
Systemic Regulatory Failure
- Manufacturing process changes without validation
- Internal regulatory alarm without public disclosure
- Removal of planned safety comparisons
- Manufacturer prior knowledge without adequate mitigation
The Regulatory Failure: From Standards to Systemic Breakdown
The Standards That Were Ignored
EMA ICH Q5B – Standards for biologics typically allow <10 ng/dose of residual DNA and explicitly forbid sequences with known oncogenic activity. 🔗 https://www.ema.europa.eu/en/ich-q5b-analysis-expression-construct-cell-lines-used-production-rdna-derived-protein-products-scientific-guideline
WHO TRS 978 – Clear guidelines for residual host-cell DNA with emphasis on fragmentation and biological inactivation.
The Transparency Gap
- No public release of Process 1 vs Process 2 comparability data
- Internal regulatory concerns concealed from public view
- Independent lab findings dismissed without GMP-grade replication
- Manufacturer patents showing prior knowledge ignored in risk assessment
Regulatory Divergence: Florida vs. Federal Agencies
The SV40 contamination revelation created an unprecedented regulatory schism:
Florida Department of Health (January 2024):
- Issued formal guidance to halt use of mRNA COVID-19 vaccines
- Cited SV40 promoter-enhancer presence as undisclosed and potentially oncogenic
- Demanded biodistribution and integration studies before continued use
- (Florida DoH, 2024 - Archived)
FDA Response (December 2023):
- Claimed SV40 fragment "lacks replication origins" and is "inactive"
- Asserted no oncogenic risk at detected levels
- No requirement for additional integration studies
- (FDA, 2023)
TGA (Australia) FOI (December 2024):
- Acknowledged SV40 promoter presence
- Denied cancer link based on "lack of large-scale epidemiological evidence"
- (TGA FOI-0070, December 2024 response)
The scientific dispute:
- FDA position: SV40 fragment is "just a promoter" without full viral genome, therefore inactive
- Critics' position: The promoter itself is the oncogenic risk via NLS-mediated integration, not viral replication
This regulatory disagreement mirrors the early HPV vaccine debates, where state-level health authorities diverged from federal guidance based on emerging safety signals.
Evidence Table: SUPPORT vs DENY
| Side | CLAIM | MECHANISM | EVIDENCE (PMID/DOI, design, N) | Main Result | GRADE | RISK | ACTION |
|---|---|---|---|---|---|---|---|
| SUPPORT | DNA contamination exceeds regulatory limits in mRNA vaccines. | Plasmid DNA fragments from E. coli production not fully removed. | Speicher et al., 2025; DOI:10.1080/08916934.2025.2551517 (27 lots analyzed) | Up to 627-fold exceedance of 10 ng/dose limit; 100% of lots contaminated. | High | Potential genotoxicity; integration risk. | Independent replication confirmed; regulatory acknowledgment required. |
| SUPPORT | SV40 promoter-enhancer sequence present in vaccine vials. | Undisclosed sequence in plasmid template. | McKernan et al., 2023/2024; Various preprints (Multiple independent labs) | SV40 promoter/enhancer detected across multiple laboratories. | High | NLS-mediated nuclear entry; oncogenic potential (IARC Group 2A). | Requires biodistribution and integration studies. |
| SUPPORT | Bacterial m⁶A methylation signature confirms E. coli origin. | Dam methylase activity leaves detectable fingerprint. | McKernan et al., 2025; Zenodo (Forensic sequencing) | N⁶-methyladenine in GATC motifs — bacterial signature confirmed. | High | Immunostimulatory; activates cGAS-STING/TLR9. | Quantify innate immune activation risk. |
| SUPPORT | RNA/DNA hybrids survive digestion and remain biologically active. | Hybrid formation protects nucleic acids from degradation. | Jessica Rose, 2024; Substack (Mechanistic analysis) | RNA/DNA hybrids resist enzymatic digestion; extend contaminant half-life. | Moderate | Prolonged exposure to contaminants; unknown integration window. | Quantify hybrid formation in vivo; assess persistence kinetics. |
| SUPPORT | Spike protein persists months post-vaccination. | m1Ψ modification stabilizes mRNA; germinal center retention. | Röltgen et al., 2022; Cell (n=14, 60-day follow-up) | mRNA and spike detected in lymph nodes ≥60 days. | Moderate-High | Prolonged antigen exposure; immune exhaustion. | Clinical correlation with adverse events needed. |
| SUPPORT | SV40 enhancer can function as somatic hypermutation targeting element. | Hijacks AID enzyme; redirects antibody diversification. | bioRxiv 2024; DOI:10.1101/2024.01.01.573752v1 (Preprint) | SV40 enhancer acts as SHM targeting element in vitro. | Moderate (mechanistic) | Potential immune disruption; oncogenic mutations. | Clinical significance requires validation. |
| DENY | DNA contamination is inert and below safety thresholds. | Fragmented DNA cannot integrate; too small for function. | Multiple independent labs (9+ confirmations) show 10-627x exceedance. | Claim contradicted by global lab consensus. | High (negative) | Dismissing evidence undermines trust. | Acknowledge exceedance; debate clinical significance. |
| DENY | SV40 promoter is "inactive" without full viral genome. | Promoter alone cannot drive oncogenesis. | FDA 2023 response; critics cite NLS-mediated integration risk. | Unresolved scientific dispute; regulatory divergence. | Moderate | Premature closure of legitimate safety question. | Florida DoH halted use; scientific resolution needed. |
| DENY | No evidence linking mRNA vaccines to cancer mortality. | Temporal association ≠ causation. | Ueda et al., 2024; PMC11077472 (7,162 excess deaths, Japan) — ⚠️ RETRACTED | Signal exists but article retracted; confounding factors acknowledged. | Invalid (retracted) | This source is no longer reliable — findings retracted by publisher. | Cancer registry surveillance with 5-10 year lag required using valid data sources. |
Key Takeaway: DNA contamination is confirmed fact. SV40 biological activity is mechanistically plausible. Population-level clinical impact remains uncertain but cannot be dismissed without proper long-term surveillance. Regulatory closure of legitimate questions undermines trust.
Contested / Active-Debate Notes
Ongoing scientific disputes where reasonable people disagree:
| Issue | Position A | Position B | Current Status |
|---|---|---|---|
| SV40 oncogenic risk | Functional promoter with NLS = integration risk | Fragment too small without replication origin | IARC Group 2A (probably carcinogenic); FDA disputes clinical relevance |
| Clinical significance of contamination | Biologically active = potential harm | Quantity too small for measurable effect | No consensus; requires integration studies |
| Japan cancer mortality signal | Causal link to mRNA vaccines | Confounding (delayed screenings, pandemic stress) | Primary source (Ueda et al., 2024) was retracted — any signal requires new independent confirmation |
| Spike persistence duration | Up to 15 months documented | Clears within months; detection artifacts | Multiple studies confirm persistence; clinical relevance debated |
| Regulatory response adequacy | Systemic failure; recalls warranted | Within acceptable safety margins | Unprecedented regulatory divergence (Florida vs FDA) |
What both sides agree on:
- DNA contamination exists and exceeds limits
- SV40 sequence is present
- More long-term surveillance is needed
- Transparency has been inadequate
Practical Biomarker Tracking
Tracking biomarkers over time provides measurable data for those concerned about vaccine exposure or experiencing persistent symptoms.
How to use: Repeat every 8–12 weeks while symptoms evolve. Look for directional trends, not single "perfect" numbers. Most advanced testing requires specialty labs or research settings.
| Panel | Biomarker | Why it helps (one-liner) | Availability |
|---|---|---|---|
| Spike Persistence | Circulating spike protein (LC-MS/MS) | Direct detection of persistent antigen | Research setting (limited availability) |
| Anti-spike antibody ratios | Persistent exposure vs declining immunity | Standard clinical labs | |
| Inflammation | hs-CRP, IL-6, TNF-α | Chronic immune activation burden | Standard labs |
| Autoimmunity | ANA, anti-phospholipid antibodies | Self-attack marker development | Standard labs |
| DNA Damage | γH2AX (phosphorylated histone) | DNA double-strand break marker | Research/specialty labs |
| 8-OHdG (8-hydroxy-2'deoxyguanosine) | Oxidative DNA damage marker | Specialty labs | |
| Immune Activation | cGAS-STING pathway markers | Foreign DNA sensing activation | Research setting |
| Cytokine panels (IFN-α/β, ISG15) | Innate immune response profile | Specialized testing | |
| Cellular Health | LDH, ferritin | General cell turnover/inflammation | Standard labs |
| D-dimer, fibrinogen | Coagulation/microclot assessment | Standard labs |
Reality check: Absence of testing does not mean absence of risk. Symptoms and clinical judgment remain important. Work with a knowledgeable clinician for interpretation.
Tiered Protocol Summary
Evidence-Informed Strategies for Those Concerned:
| Tier | Evidence Level | Best For | Avoid In | Approach |
|---|---|---|---|---|
| Tier 1 - Awareness | High (documented facts) | Everyone | — | Stay informed; monitor symptoms; report adverse events |
| Tier 2 - Monitoring | Moderate | Post-vaccine symptoms; high-risk individuals | — | Track biomarkers (above); document timeline; seek knowledgeable clinicians |
| Tier 3 - Supportive Measures | Low-Moderate (mechanistic) | Persistent symptoms | Unproven treatments | Consider autophagy support (fasting, spermidine, quercetin) under guidance |
| Tier 4 - Avoidance | Variable | Personal choice | Medical coercion | Individual risk assessment based on age, health status, prior exposure |
Evidence note: Most interventions for spike persistence or DNA contamination effects remain investigational. Clinical outcome data are pending. Use caution, work with qualified clinicians.
Plain Talk — Real-World Language
If technical details feel overwhelming, start here.
The Short Version (30 seconds)
mRNA vaccines were a brilliant engineering solution that won a Nobel Prize. But something went wrong between the clinical trials and mass production. The vaccine billions received was not the same one tested in trials. It contained bacterial DNA with active viral promoters—contamination that regulatory scientists privately called alarming but publicly dismissed.
This DNA is not inert. It can trigger chronic immune activation and may interfere with how your body makes antibodies. The spike protein itself persists longer than expected in some people.
What this means: We are in uncharted territory. The "safe and effective" narrative does not match the documented reality. More research is needed. More transparency is required. Your health autonomy matters.
A Simple Analogy
Think of mRNA vaccines like a car:
The engineering triumph = A revolutionary engine that wins awards
The problem = The production version was built differently than the prototype, with unauthorized parts that engineers privately warned about but manufacturers used anyway
The result = Cars are being recalled, but regulators are saying "drive it anyway, it is probably fine" while some mechanics are finding dangerous problems
What you should do = Get informed, ask questions, and make your own choices based on complete information
How This Can Feel Day to Day
People experiencing persistent symptoms after vaccination report:
- Fatigue that does not improve with rest
- Brain fog or difficulty concentrating
- Racing heart or blood pressure changes
- New allergies or food sensitivities
- Muscle and joint pain
- "Flare-ups" triggered by exertion
- Feeling "wired but tired"
These are real symptoms. They deserve real investigation, not dismissal.
FAQ: Quick Answers
Should I get boosted if I have had reactions?
That is your decision. But consider: (1) Prior reactions increase risk of future reactions; (2) No data exists on safety for those with existing complications; (3) The burden of proof should be on demonstrating safety, not assuming it.
Can I test for DNA contamination or spike persistence?
LC-MS/MS testing can detect spike protein in research settings. Most clinical labs do not offer this. Antibody ratios and inflammatory markers provide indirect clues. Specialty testing is emerging but not widely available.
What about natural immunity vs. vaccination?
Natural infection provides broader mucosal immunity and longer-lasting memory cell responses than mRNA vaccination. Hybrid immunity (infection + vaccination) shows strongest responses in some studies. Individual circumstances vary.
Is the SV40 contamination the same as polio vaccine contamination?
Different context, same virus family. SV40 in polio vaccines (1955-1963) was later linked to rare cancers. The mRNA SV40 fragment is a promoter-enhancer sequence, not the whole virus. Both represent unintended biological contaminants with uncertain long-term effects.
What can I do if I am experiencing symptoms?
- Document everything (timeline, symptoms, tests)
- Seek clinicians who take post-vaccine injuries seriously
- Track biomarkers (inflammation, autoimmunity, clotting)
- Consider supportive care (autophagy support, detox support)
- Connect with others experiencing similar issues
Will this give me cancer?
We do not know. Early signals exist (Japan excess mortality 2022). Biological mechanisms are plausible (SV40 integration, chronic inflammation). But solid tumor development typically takes 5-10 years. What we know: the risk is not zero. What we do not know: the actual magnitude. That is why long-term surveillance matters.
Methodology & Limits
This article synthesizes evidence from:
- Peer-reviewed studies (PMIDs and DOIs provided)
- Regulatory documents (FOI releases, agency responses)
- Manufacturer patent filings
- Independent laboratory analyses
- Clinical case reports
Scope:
- Does not assert population-level causation where temporal associations exist
- Clearly distinguishes between documented facts, plausible mechanisms, and speculative concerns
- Acknowledges legitimate scientific disputes and regulatory disagreements
What readers can expect:
- Direct citations to primary literature
- Clear distinction between confirmed findings and mechanistic hypotheses
- Transparency about uncertainties and open questions
- Multiple perspectives on contested issues
What this article is NOT:
- Medical advice or diagnosis
- A blanket condemnation of all mRNA technology
- Anti-vaccine propaganda (it is pro-transparency and pro-accountability)
- A substitute for professional medical care
🔗 Related Investigations
For readers who want to explore specific aspects of this story in more depth:
SV40 DNA Signals in COVID-19 mRNA Vaccine Vials
Comprehensive analysis of independent laboratory findings across eight international teams confirming plasmid DNA and SV40 promoter contamination.Pfizer Process 1 vs Process 2: What Changed, What's Measured, and What Remains Uncertain
Investigation into the manufacturing changes between clinical trial and commercial vaccine lots, and the removed safety comparisons.DNA Contamination & mRNA Vaccine Biology: Curated Reference Roadmap
A curated, citable collection of primary sources, regulatory documents, and scientific studies on DNA contamination and related biological mechanisms.
Conclusion: From Engineering Triumph to Biological Trap
The emerging picture reveals a perfect storm of intersecting factors:
What we know:
- DNA contamination is real, widespread, and confirmed by global scientific consensus
- The contamination is biologically active with bacterial methylation and functional SV40 promoters
- Internal regulators were alarmed while maintaining public assurances
- Manufacturers documented these risks years before the pandemic
- Multiple biological mechanisms exist for potential harm
What we don't know:
- The full clinical impact of chronic DNA exposure via LNPs
- The population-level effects of somatic hypermutation disruption
- The long-term consequences of persistent spike protein and immune activation
- Whether early cancer mortality signals (Japan 2022) represent true causality or confounding
- The integration frequency in human tissues and which cell types are most affected
The burden of proof has decisively shifted. It's no longer sufficient to say "no evidence of harm"—we need robust evidence of safety in light of these documented contamination issues, identified biological mechanisms, and regulatory transparency failures.
The story of mRNA vaccines is evolving from one of pure triumph to a complex case study in how engineering solutions can create unintended biological consequences when combined with quality control failures and regulatory breakdowns.
How to Cite
Anthony T. The Stability Trap: How mRNA Vaccine Engineering and DNA Contamination Created a Perfect Storm. Published 18 Oct 2025. Updated 29 Jan 2026. Available at: measslainte.com (accessed [insert date]).
This analysis synthesizes emerging scientific evidence from peer-reviewed studies, regulatory documents, manufacturer patents, and independent laboratory analyses. It is presented for informational and discussion purposes to advance scientific transparency and public understanding. It is not medical advice. Consult healthcare professionals for medical decisions.