The Discovery That Changed Everything
In early 2023, something extraordinary happened in a Massachusetts laboratory. Researcher Kevin McKernan, running routine quality checks, found DNA fragments in mRNA COVID-19 vaccines that shouldn't have been there. What began as a simple contamination check soon revealed a much larger story about pharmaceutical manufacturing, regulatory oversight, and the fundamental question: What exactly were we injecting into our bodies?
This isn't just about technical specifications—it's about the discovery of plasmid DNA, SV40 viral sequences, and manufacturing residuals in products given to billions under the banner of "safe and effective."
The Manufacturing Change: Process 1 vs Process 2
The Unannounced Switch
The vaccine used in clinical trials—the one that demonstrated safety and efficacy—was manufactured under "Process 1." The version billions received came from "Process 2." The fundamental question remains: Were they actually the same product?
The Disappearing Safety Check
Pfizer's original trial design included a crucial safeguard: a cohort of participants would receive both Process 1 and Process 2 material for direct comparison. This wasn't an optional extra—it was fundamental science.
Then, in September 2022, Protocol Amendment 20 removed this objective (Archive of Protocol C4591001 Amendment 20). The planned safety comparison vanished just as Process 2 dominated global supply.
When researchers filed Freedom of Information requests asking how equivalence was demonstrated, regulators acknowledged the question but provided no analytical data (MHRA FOI 23/510).
For detailed analysis of manufacturing changes and their implications, see Pfizer Process 1 vs Process 2: What Changed, What's Measured, and What Remains Uncertain
The Contamination Findings: Global Independent Lab Consensus
The Initial Discovery
Kevin McKernan's team found DNA fragments in both Pfizer and Moderna vaccines, reporting their findings in a detailed OSF preprint that would spark global concern (McKernan et al., 2023).
The Global Replication
Multiple laboratories across North America, Europe, and Asia have now confirmed the findings, creating an undeniable scientific consensus:
- Kevin McKernan (USA): qPCR estimates of 10-60 ng plasmid DNA per dose, with sequencing confirming SV40 promoter/enhancer (OSF Preprint)
- Tomonori Nitta (Japan): Peer-reviewed study finding 1-18 ng DNA per dose with SV40 promoter detected (Frontiers in Immunology 2024)
- Phillip Buckhaults (USA): 1-6 ng DNA per dose with SV40 sequences confirmed in legislative testimony (South Carolina Senate)
- David Speicher et al. (Canada/Australia): Analysis of 27+ vials showing 0.22-510 ng DNA range (OSF Preprint)
- König & Kirchner (Germany): Reported 3,600-5,340 ng/dose (500x regulatory limit)
- Kämmerer et al. (Germany): Confirmed SV40 promoter/enhancer in LNPs
- Raoult et al. (France): Found full plasmid recovery in vials
The Critical Pattern
Despite methodological differences, multiple independent labs consistently detect:
- Plasmid DNA contamination above the 10 ng/dose regulatory benchmark
- SV40 promoter/enhancer sequences present in final products
- Lot-to-lot variability suggesting inconsistent manufacturing control
For comprehensive laboratory findings and methodology details, see SV40 DNA Signals in COVID-19 mRNA Vaccine Vials: What Three Independent Labs Reported
🚨 Health Canada's Internal Crisis: The Regulatory Smoking Gun
Internal emails obtained through investigative work by Scoops McGoo reveal a regulatory crisis concealed from public view:
The Unplanned Discovery
- Pfizer's Admission: "Yes, because Pfizer did not identify the presence of SV40 promoter enhancer on the plasmid template used to produce mRNA" (Email Pg. 235)
- Quality Control Failure: Manufacturer's own controls failed to detect SV40 sequence
Regulatory Alarm Bells
- "SV40 must be avoided!" (Pg. 66) - Clear safety principle violation
- "Remedy the situation before Fall 2024 vax campaign" (Pg. 141) - Urgent timeline for resolution
- "They do not seem to care much at this moment" (Pg. 151) - Frustration with Pfizer's response
Scientific Concern
- "Fragment size is related to the probability of integration" (Pg. 205) - Direct genotoxicity discussion
- "References: None" beside "minimal safety risk" claim (Pg. 203) - Unsubstantiated safety assurance
🧬 The Somatic Hypermutation Threat: Immune System Sabotage
A January 2024 preprint reveals the most concerning mechanism yet: SV40 enhancer functions as a somatic hypermutation targeting element (bioRxiv).
Understanding the Mechanism
- Somatic Hypermutation (SHM): Process where B-cells "fine-tune" antibodies for better pathogen recognition
- AID Enzyme: "Master catalyst" that enables antibody diversity through targeted mutation
- SV40 Hijacking: Can redirect SHM machinery, potentially causing:
Potential Consequences
- Impaired Antibody Responses: Disrupted SHM → reduced infection fighting capability
- Oncogenic Mutations: Aberrant AID targeting → cancer-causing DNA changes
- Autoimmune Development: Misdirected hypermutation → self-attacking antibodies
As immunologist Jessica Rose summarizes: "If we have impaired antibody responses, we have a broken immune system."
💡 Manufacturer Prior Knowledge: The Moderna Patent Admissions
Modernas's own intellectual property reveals they understood the risks years before COVID-19:
Explicit Warnings in Patent Documentation
- "DNA fragments resulting from the mRNA production process need to be removed"
- "Because the remaining DNA fragments may cause a patient to develop cancer"
- "qPCR can't measure small DNA fragments, yet we have to use another method to quantify them"
- "Some of the introduced DNA may be incorporated into the genome of the cell and inherited by the offspring"
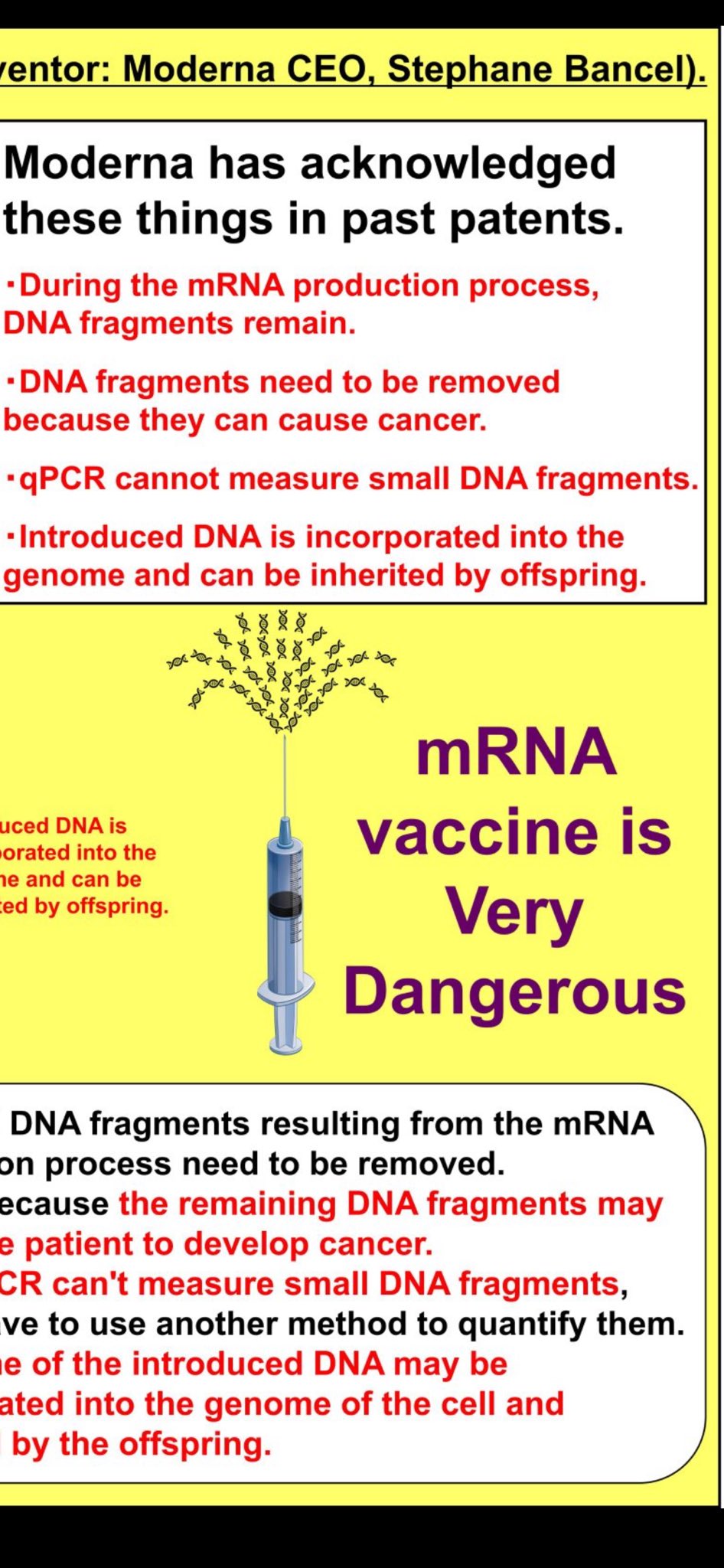
These admissions demonstrate that DNA contamination risks were well-understood, documented, and explicitly warned against in manufacturer's own patent filings years before the pandemic vaccine rollout.
The Biological Mechanisms: How Contamination Could Cause Harm
Reverse Transcription Concerns
A 2022 study by Aldén et al. found that BNT162b2 mRNA can be reverse-transcribed into DNA in human liver cells (Current Issues in Molecular Biology). This challenges the long-standing assumption that mRNA cannot integrate into human DNA.
Immune System Impacts
Multiple studies documented unexpected immunological effects:
- IgG4 Class Switch: Irrgang et al. found that repeated mRNA vaccination causes a class switch toward less protective IgG4 antibodies (PubMed, 2023)
- Spike Protein Persistence: Patterson et al. detected SARS-CoV-2 spike protein in monocytes up to 15 months post-infection (Frontiers in Immunology)
Autopsy Evidence
Disturbing pathological findings emerged from post-vaccination autopsies:
- Multifocal Necrotizing Encephalitis: Mörz documented brain inflammation after BNT162b2 vaccination (Vaccines, 2022)
- Myocarditis Pathology: Schwab et al. characterized myocarditis histopathology following vaccination (PMC, 2023)
- Adolescent Deaths: Gill et al. reported autopsy findings in two teenagers who died after Pfizer vaccination (Archives of Pathology & Laboratory Medicine)
The Regulatory Failure: Systemic Transparency Gaps
Established Safety Standards
The International Council for Harmonisation (ICH) has clear guidelines for residual DNA in biological products (ICH Q6B). The established limit is 10 ng/dose, with multiple independent measurements reportedly exceeding this threshold.
The Transparency Crisis
When researchers sought clarity through formal channels, they encountered systematic opacity:
- UK's MHRA acknowledged FOI requests about Process 1 vs Process 2 equivalence but released no analytical data
- Regulators maintain authorized lots meet specifications but have not published raw lot data
- No GMP-grade independent replication has been facilitated despite multiple lab confirmations
The Scientific Community's Response
The topic remains controversial, with one paper by König & Kirchner receiving an "Expression of Concern" despite its methodological rigor (MDPI Methods Protoc).
Traffic-Light Snapshot: Safety Claims vs Evidence
"The manufacturing change was rigorously validated"
- Claim: Process 2 equivalent to Process 1
- Evidence: Comparison cohort removed from trial; FOI requests yield no data; SV40 sequence was unexpected finding; Multiple independent labs find DNA contamination in Process 2 vials
- Verdict: ❌ Validation failure - No public proof despite global lab confirmations
"DNA fragments are within safe limits"
- Claim: All lots meet regulatory standards
- Evidence: Independent labs report variable levels; some exceed 10 ng/dose; SV40 shown to disrupt somatic hypermutation
- Verdict: ❌ Unacceptable risk - mechanism identified for immune system harm
"SV40 sequences are biologically inert"
- Claim: Viral fragments have no functional activity
- Evidence: Historical concerns about SV40; promoter/enhancer sequences present; Moderna patents acknowledge cancer risk; SV40 targets somatic hypermutation machinery
- Verdict: ❌ Biologically active - multiple mechanisms of harm identified
"mRNA cannot integrate into human DNA"
- Claim: Genetic material remains separate from genome
- Evidence: Aldén et al. showed reverse transcription in liver cells; Health Canada emails discuss integration probability
- Verdict: ❌ Integration possible - experimental evidence challenges assumption
The Human Cost: Beyond Technical Specifications
Behind the laboratory findings and regulatory debates are real human stories:
- The families of teenagers who died unexpectedly after vaccination
- The patients with unexplained inflammatory conditions
- The doctors observing patterns they can't explain through conventional medicine
- The researchers facing professional backlash for asking difficult questions
What Proper Accountability Would Look Like
- Full Data Transparency: Release all manufacturing quality control data and comparability studies
- Independent Auditing: Multi-laboratory analysis of retained vaccine lots using validated methods
- Comprehensive Safety Review: Examine adverse event patterns in context of manufacturing variations
- Regulatory Reform: Ensure future emergency products undergo the same rigorous quality control as conventional medicines
🔗 Related Investigations
For readers seeking deeper analysis of specific aspects:
The Stability Trap: How mRNA Vaccine Engineering and DNA Contamination Created a Perfect Storm
Comprehensive analysis of how mRNA stability modifications intersect with DNA contamination to create biological risks.SV40 DNA Signals in COVID-19 mRNA Vaccine Vials
Detailed laboratory findings and methodological analysis from eight independent research teams.Pfizer Process 1 vs Process 2: What Changed, What's Measured, and What Remains Uncertain
Investigation into manufacturing changes between clinical trial and commercial vaccine lots.
The Unanswered Question
When you received your mRNA COVID-19 vaccine, were you told about the plasmid DNA fragments? The SV40 sequences? The variable manufacturing quality? The potential threat to your immune system's antibody diversity?
The fundamental compact between pharmaceutical companies, regulators, and the public relies on transparent communication about product quality and potential risks. The DNA contamination story suggests this compact was broken.
As one researcher privately confessed: "We're finding things that, according to the official specifications, shouldn't be there. The question isn't whether there's contamination—it's whether it matters."
This investigation exists because independent scientists refused to look away when they found unexpected results. In a properly functioning regulatory system, these questions would have been asked—and answered—long before products reached billions of people.